LymphActivist's Site
Dedicated to Lymphedema Patients and the Therapists Who Treat Them
10671 Baton Rouge Avenue
Porter Ranch, CA 91326-2905
September 26, 2016
By E-Mail
Centers for Medicare & Medicaid Services
Director, Coverage and Analysis Group
7500 Security Boulevard
Baltimore, MD 21244
A Formal Request for a National Coverage Determination
Coverage of Lymphedema Compression Treatment Items as Prosthetic Device Benefits
The attached request is being submitted in accordance with CMS-3284-N, Medicare Program: Revised Process for Making National Coverage Determinations. This complete formal request is being submitted by an individual independent patient advocate who represents Medicare beneficiaries in their appeals of denials of coverage for their medically necessary, physician-prescribed lymphedema compression items. It presents evidence to show that compression bandage systems, garments, devices and supplies used in the compression therapy of lymphedema meet all of the coverage criteria for prosthetic device benefits per Section 1861(s)(8) of the Act as defined in the Medicare Benefit Policy Manual (MBPM), CMS Pub. 100-02, Chapter 15, Section 120 Prosthetic Devices.
The substance of this evidentiary material has been seen by CMS on a number of occasions, notably in a presentation to the Coverage and Analysis Group by Dr. Wade Farrow, MD and Mr. Robert Weiss, MS on October 24, 2006, to the HCPCS Workgroup by Mr. Weiss on May 1, 2007, and on behalf of Medi USA on May 24, 2011 and May 9, 2012. In the last decade our knowledge of the lymphatic system and the benefits of early treatment have advanced tremendously, and the variety of compression items for the treatment of lymphedema has grown.
Since 2004 the number of ALJ reversals of denials of these items have also grown as the medico-legal arguments presented in this request have been reviewed by more and more Administrative Law Judges. On November 18, 2009 a MEDCAC Lymphedema Expert Panel determined that there is moderate to good evidence that these compressive protocols, with and without adjuvant sequential pneumatic compression, will result in an improvement in the health of lymphedema patients. Recent studies in peer-reviewed journals have also indicated that the early treatment of lymphedema results in a substantial decrease in the cases of lymphedema-related cellulitis, more than offsetting the cost of coverage for these items.
The target Medicare population comprises beneficiaries with diagnosed primary or secondary lymphedema ICD-10-CM Codes Q82.0, I89.0, I97.2 and I97.89, and beneficiaries with heightened risk or early signs of lymphedema after surgery, radiotherapy, physical trauma or infection. These compression items are used by health care providers, predominantly by therapists as part of their Part A or Part B intensive phase lymphedema treatment (e.g. compression bandages applied as part of CPT 97140), and by beneficiaries as part of their Part B daily and nightly home management of this chronic disease.
There is general agreement among lymphedema researchers, clinicians, and therapists that compression is the key protocol in the treatment and management of chronic lymphedema. Day and night compression is often required to prevent swelling of a lymphedematous limb. Prevention of swelling retards or eliminates pathological tissue changes such as fibrosis and fat accumulation that cause a progressive worsening of lymphedema. Recent studies have shown that early intervention can prevent development of clinical lymphedema, at which time it becomes more difficult and expensive to treat. Studies also show that a reduction of lymphedema greatly lowers the risk for lymphedema-related cellulitis, a skin infection frequently requiring hospitalization.
In spite of the evidence that treatment of lymphedema by compression will result in an improvement in the health of lymphedema patients, Medicare does not cover the cost of the compression bandages, garments, devices and supplies. The reason frequently given is that there is no covered benefit category in which they fit. It is the intent of this formal request for a national coverage determination to question this statement, and to propose that these materials, when used in the medical treatment of lymphedema, meet all of the statutory [Section 1861(s)(8) of the Act] and policy [MBPM, CMS Pub. 100-02, Chap. 15, Sect. 120] coverage criteria for “prosthetic devices”, and, despite absence of an NCD or LCD, are covered by Medicare.
After a summary and background in Sections 1 and 2, the evidence document examines the nature of lymphedema, its relationship to cellulitis, and its national prevalence and burden in Sections 3 and 4. The value of early detection and treatment of lymphedema is stressed. Current protocols for treatment of lymphedema are covered in Section 5, with Medicare coverage and non-coverage of these treatment protocols detailed. The unanticipated effects of a 2006 administrative change in HCPCS coding (Appendix A) with its disastrous effect on the lymphedema patient community are discussed. Section 5 also introduces the definition of the “prosthetic device” benefit category that has been the basis for scores of Administrative Law Judges’ reversals of Medicare Contractors’ coverage denials of lymphedema compression garments. A description of the various types of compression items is found in Section 6 with explanations of how they are used in the medical treatment of lymphedema.
Section 7 is the core of this proposal. This section reviews the statutory and policy bases for Medicare coverage, with special attention to the flow down of coverage requirement documentation from the Social Security Act for each of the four durable medical equipment, prosthetics, orthotics and supplies (DMEPOS) benefit categories. Particular attention should be given to the discussions pertaining to benefit categories, since it is the misunderstanding of this subject that leads to Durable Medical Equipment Medicare Administrative Contractors’ (DME MACs) illogical denials of coverage of prosthetic devices because they do not meet the coverage criteria for surgical dressing or durable medical equipment benefits.
After defining the four DMEPOS benefit categories, focus is changed in Section 7 to the particular benefit category of prosthetic devices (not to be confused with the prosthetics benefit category). It is shown how compression items, when used in the medical treatment of lymphedema, meet the coverage criteria for prosthetic devices, as defined in the MBPM, CMS Pub. 100-02, Chap. 15, Sect. 120 Prosthetic Devices. Additional medical and clinical support of this position is found in Appendix B.
Section 8 suggests corrections and changes to CMS documentation to support this proposed change, and Section 9 lists references and citations. Appendix C presents a preliminary proposed matrix of lymphedema compression items suggested in 2007 to be added as L-Codes in the HCPCS Code Book.
Robert Weiss, M.S.
Independent lymphedema patient advocate
A FORMAL REQUEST FOR A NATIONAL COVERAGE DETERMINATION— COVERAGE OF LYMPHEDEMA COMPRESSSION TREATMENT ITEMS AS PROSTHETIC DEVICE BENEFITS
1. SUMMARY
Lymphedema, a common sequela of cancer treatment, affects some 3-6 million Americans. Without treatment and management it is a chronic, progressive condition that often results in recurrent infection and ultimately disability. Diagnosis and pathology of lymphedema is discussed, and the current medical treatment protocols are described. The essential role of compression in the medical treatment of lymphedema is highlighted. A summary of the criteria for coverage under the Medicare Program is introduced for the purpose of demonstrating that denial of claims for the compression bandage systems, compression garments, devices and supplies is not based on Medicare law, statute or policy, and that there is a basis for coverage of these items when used in the customary treatment of diagnosed lymphedema.
The only currently accepted treatment of lymphedema from all causes (primary as well as secondary) is “complete/complex decongestive therapy” with or without sequential intermittent pneumatic compression [MEDCAC 2009]. The key essential protocol for lymphedema treatment in the clinic as well as in home settings is compression. The materials used to implement lymphedema compression therapy are not currently covered by Medicare despite the fact that enabling statutes and policies already exist. The purpose of this request is to present evidence to support opening of a National Coverage Analysis leading to the coverage of compression garments used in the treatment of lymphedema as prosthetic devices in accordance with the Medicare Benefit Coverage Manual, CMS Publication 100-02, Chapter 15, §120.
The medico-legal logic has been considered by scores of U.S. Administrative Law Judges, the majority of whom have held that these compression bandage systems, compression garments and compression devices, when they are used in the compression therapy for diagnosed lymphedema, are reasonable and medically necessary, and meet the Medicare coverage criteria for “prosthetic devices”.
ROADMAP
| 1. SUMMARY | 1 |
2. BACKGROUND | 2 |
3. THE NATURE OF LYMPHEDEMA | 3 |
4. THE NATIONAL BURDEN OF LYMPHEDEMA | 6 |
5. MEDICARE COVERAGE OF THESE ITEMS | 9 |
6. DESCRIPTION OF THE ITEMS OF SERVICE | 11 |
7. BENEFIT CATEGORY IN WHICH COMPRESSION ITEMS FALL | 21 |
8. CLARIFICATIONS/CHANGES TO EXISTING MEDICARE DOCUMENTATION | 32 |
9. REFERENCES | 34 |
APPENDIX A. 2005 CHANGE OF HCPCS CODING FOR COMPRESSION ITEMS | 40 |
APPENDIX B. HOW LYMPHEDEMA SUPPLIES ARE COVERABLE BY SSA | 42 |
APPENDIX C. 2007 PROPOSED EXPANSION OF HCPCS CODES | 43 |
Table 1. Flow down of DMEPOS requirements | 23 |
Table 2 Mechanisms and effects of compression therapy in lymphoedema, Partsch 2006 | 29 |
2. BACKGROUND
The last decade has brought a burgeoning of lymphatic system research that is changing the very fundamentals of our understanding of this complex body system. This new research is exposing how the lymphatic system is or may be involved in a variety of clinical conditions including edema, infection, cardiovascular disease, autoimmunity, Crohn’s disease, obesity, cancer and lymphedema. In the new view, all chronic edema indicates an inadequacy or failure of the lymphatic system, and it is vital for the clinician to determine the source of the edema, whether primary impairment of the lymphatics or secondary overload of the lymphatics due to another systemic failure.
Pathology of Lymphedema. Many pathological mechanisms of impaired lymph drainage are being elucidated, including failure of initial lymphatic absorption, aberrant lymphangion smooth muscle function, faulty lymphatic valve operation and faulty control of lymphangion smooth muscle pumping. Lymphedema is more than "a blockage in your lymphatic system"[Mayo 2015] as incorrectly defined in some authoritative sources today.
Role in Infection, Immunity and Inflammation. Another vital role of the lymphatic system is host defense, with new knowledge of the role of the lymphatics in infection, immunity and inflammation. The greatly elevated rate of infection associated with lymphedema of all forms, and potential associations of lymphedema with changes in immunity are being defined. "The range of diseases associated with lymphatic dysfunction causing disturbed immunity is likely to be extensive." Possible involvement of lymphatic dysfunction has been discovered in inflammatory bowel disease, particularly Crohn’s disease, pointing to immune deficiency rather than autoimmunity as the functional cause. [Mortimer 2014]
Role in Lipid Pathologies. The relationship between fat and lymphatics focuses on the role of the lymphatic system in the generation, absorption and disposition of fat. Much is still not known, but examples are given not only of the role of the lymphatics in absorption and transport of fat by intestinal lacteals and the fat content of persistent lymphatic tissue, but of the other side of the coin-- the influence of obesity on the incidence of lymphedema. The role of the lymphatic function in lipid pathologies such as obesity, diabetes, hypercholesterolemia and atherosclerosis "remain to be elucidated". [Mortimer 2014]
Importance of early Intervention. An important theme has been arising in the last ten years that may be the key to cost savings and improvement in patient health—that early conservative intervention may prevent lymphedema and drastically reduce lymphedema-related infections. An early intervention program of manual lymphatic drainage (MLD) can potentially prevent the onset of lymphedema in patients surviving a wide spectrum of breast cancer treatments [Zimmermann 2012]. This study demonstrated that regardless of the surgery type and the number of lymph nodes removed, early manual lymph drainage (MLD) effectively prevented lymphedema of the arm on the operated side. It suggests that MLD administered soon after surgery for breast cancer should be considered for the prevention of lymphedema. This confirms recommendations for immediate MLD to regenerate damaged lymphatic vessels promptly and create lymphatic and venous–lymphatic connections before the occurrence of skin tissue changes and the presentation of clinical lymphedema, and before the need presents for compression [Földi 1998].
These studies confirmed results by other researchers on edema prevention after treatment of breast cancer. Significantly fewer women receiving physiotherapy developed clinically important lymphedema after one year when compared with controls [Torres-Lacomba 2010]. A physiotherapy program including exercises and progressive educational strategies may reduce the occurrence of secondary lymphedema two years after surgery [Box 2002]. And a prospective surveillance and early intervention using off-the-shelf elastic compression sleeves effectively treated subclinical lymphedema and prevented clinical lymphedema [Stout-Gergich 2008]. In a later study Stout demonstrated that the perspective surveillance model is a potential cost-saving protocol for breast cancer-related lymphedema [Stout 2012].
Early identification of the signs and symptoms of lymphedema should be integral to the management of all patients who have received surgery and/or radiation, and are thus at high risk. When treated in the earliest stages, complications of this condition may be minimized [Lawenda 2010]. In one study, treatment of lymphedema with a pneumatic compression device (PCD) was associated with decreases in rates of hospitalizations (45% to 32%, p<0.0001), outpatient hospital visits (95% to 90%, p<0.0001), cellulitis diagnoses (28% to 22%, p=0.003), and physical therapy use (50% to 41%, p<0.0001). The average baseline health care costs were high ($53,422) but decreased in the year after PCD acquisition (-$11,833, p<0.0001) [Brayton 2014].
3. THE NATURE OF LYMPHEDEMA
Lymphatic System Anatomy [Britannica 2015] Lymphatic system, a subsystem of circulatory system in the vertebrate body that consists of a complex network of vessels, tissues, and organs. The lymphatic system helps fluid balance in the body by collecting excess fluid and particulate matter from tissues and depositing them in the bloodstream. It also helps defend the body against infection by supplying disease-fighting cells-- lymphocytes.
The lymphatic system can be thought of as a drainage system needed because, as blood circulates through the body, blood plasma leaks into tissues through the thin walls of the capillaries. The portion of blood plasma that escapes is called interstitial or extracellular fluid, and it contains oxygen, glucose, amino acids, and other nutrients needed by tissue cells. Although most of this fluid seeps immediately back into the bloodstream, a percentage of it, along with the particulate matter, is left behind. The lymphatic system removes this fluid and these materials from tissues, returning them via the lymphatic vessels to the bloodstream, and thus prevents a fluid imbalance that would result in the organism’s death.
The fluid and proteins within the tissues begin their journey back to the bloodstream by passing into tiny lymphatic capillaries that infuse almost every tissue of the body. Only a few regions, including the epidermis of the skin, the mucous membranes, the bone marrow and the central nervous system, are free of lymphatic capillaries [or collectors], whereas regions such as the lungs, gut, genitourinary system, and dermis of the skin are densely packed with these vessels. Once within the lymphatic system, the extracellular fluid, which is now called “lymph”, drains into larger vessels called the lymphatics [lymph vessels or lymphangions]. These vessels converge to form one of two large vessels called lymphatic trunks, which are connected to veins at the base of the neck. One of these trunks, the right lymphatic duct, drains the upper right portion of the body, returning lymph to the bloodstream via the right subclavian vein. The other trunk, the thoracic duct, drains the rest of the body into the left subclavian vein. Lymph is transported along the system of vessels by muscle contractions [and external forces], and one-way valves prevent lymph from flowing backward. The lymphatic vessels are punctuated at intervals by small masses of lymph tissue, called lymph nodes that remove foreign materials such as infectious microorganisms from the lymph filtering through them.
Pathophysiology of Lymphedema. Lymphedema, also called “lymph stasis”, can be defined as the tissue fluid accumulation that arises as a consequence of impaired lymphatic drainage. Reduction of lymphatic flow can result from either congenital or acquired anomalies of lymphatic outflow. Although lymphedema usually affects one or more of the limbs, its effects can manifest in other organs. Whatever the pathogenesis, it is most often a chronic, unrelenting condition, posing long-term physical and psychological difficulties for the patient and a complex therapeutic challenge for the physician [Szuba 1998].
Lymphedema is a chronic progressive condition of lymphatic insufficiency that leads to swelling and serious infections due to the immune defect induced by loss of lymphatic function. Lymphedema requires lifelong treatment [Hutchison 2010]. Far from being incurable, the disease now has many treatment options that have demonstrable efficacy for the reduction of edema volume and the prevention of fluid accumulation. On the other hand, if the treatment regimen is abandoned, continuous accumulation of tissue fluid will ensue, exacerbated by recurrent infection, with resultant massive edema, grossly impaired limb function, psychosocial disability and life-threatening infectious or malignant complications [Szuba 1998].
Time Course of Lymphedema [Bar Ad 2009]. In a study cohort of 1,713 consecutive Stage I or II breast cancer patients who underwent breast conservation therapy including axillary staging followed by radiation, arm lymphedema was documented in 266 (16%) of the patients. One hundred and nine patients, 6% of the overall group and 40% of the patients with arm lymphedema, presented with mild arm lymphedema, defined as a difference of 2 cm or less between the measured circumferences of the affected and unaffected arms. Among the 109 patients with mild arm lymphedema at the time of arm lymphedema diagnosis, the rate of freedom from progression to more severe lymphedema was 79% at 1 year, 66% at 3 years, and 52% at 5 years. The patients who were morbidly obese, had positive axillary lymph nodes, or received supraclavicular irradiation at the time of breast cancer treatment were at higher risk of progression from mild arm lymphedema to more severe edema.
Mild arm lymphedema, generally considered to be a minor complication after breast conservation treatment for breast cancer, was associated with a risk of progression to a more severe grade of arm lymphedema in a substantial fraction of patients.
Cellulitis-Lymphedema Cycle [Damstra 2008]. Infection of the skin and lymphatic system (cellulitis/lymphangitis) is a major cause of lymphedema. It is also a major result of lymphedema [Stoberl & Partsch 1987]. There is evidence that there is suppression of immune competence in a lymphedematous limb [Mallon 1997]. Some 10-15% of lymphedema patients experience infections each year [Swenson 2002, Kasseroller 1998]. Research described by Dr. Kitamura at the 2010 meeting of the International Lymphoedema Framework in Brighton, England on the incidence of cellulitis in Japan among her breast cancer patients showed that 135/656 (20.6%) breast cancer survivors developed lymphedema, and of these 135 lymphedema patients 72 (53.3%) experienced recurrent cellulitis. The Kitamura figure of 11% (53.3%x20.6%) falls within the earlier figures of Swenson and Kasseroller. Treatment of lymphedema has been shown to decrease the frequency of infections [Földi 1996, Boris 1997, Ko 1998].
In a 2008 study Damstra concluded: Erysipelas (cellulitis) is often presumed to be purely infectious in origin, with a high rate of recurrence and a risk of persistent swelling due to secondary lymphoedema. In this study, we show that patients presenting with a first episode of erysipelas often have signs of pre-existing lymphatic impairment in the other, clinically non-affected, leg. This means that sub-clinical lymphatic dysfunction of both legs may be an important predisposing factor. Therefore, we recommend that treatment of erysipelas should focus not only on the infection but also on the lymphological aspects, and long-standing treatment for lymphoedema is essential in order to prevent recurrence of erysipelas and aggravation of the pre-existing lymphatic impairment. A similar conclusion by Stoberl 1987 is described by Burdette in 2005 "The investigators concluded that these data suggested that lymphedema was a predisposing factor in the cellulitis and was not caused by the infection. In another study the lymphatic drainage of 30 patients with recurrent cellulitis was studied via lymphoscintigraphy. Seventy-seven percent (23 out of 30) were found to have significant lymphatic abnormalities correlating well with the aforementioned data that show the major role of lymphedema in recurrent disease."
Signs and Symptoms of Lymphedema. Signs and symptoms of lymphedema include non-pitting edema, skin changes such as peau d’orange, pinkish-red skin discoloration, hyperkeratosis, dermatitis, eczema, ulceration, varicosity, lymph vesicles, drainage of fluid, clear or milky, or yellow discoloration or other abnormalities of the nails. The presence of Stemmer sign (skin fold of toes or fingers can barely be lifted), squaring of the toes, or puffiness of the forefoot (buffalo hump) can be noted. Such conditions/findings should be documented with appropriate clinical photos taken. Severity of pain (by using a visual analogue pain scale) and disability (by using a quality of life questionnaire) documentation at baseline is important to classify disease burden for each patient and in order to evaluate the effectiveness of any treatment. [Lee 2014]
Value of Early Detection and Treatment in Preventing Lymphedema [Laidley 2016]. With improved survivorship, the prevalence of breast cancer-related lymph-edema (BCRL) continues to increase, leading to impairment of a patient’s quality of life. While traditional diagnostic methods are limited by an inability to detect BCRL until clinically apparent, bioimpedance spectroscopy (BIS) has been shown to detect subclinical BCRL. The purpose of this study was to evaluate the role of BIS in the early detection of BCRL, as well as assessment of response to BCRL treatment.
A retrospective review of 1133 patients (average age 56.2 years) was conducted. Patients were treated for breast cancer between November 2008 and July 2013 at 2 surgical practices, with mean follow-up of 21.7 months. Chart review identified 326 eligible patients who underwent L-Dex bioimpedance spectroscopic measurements before and after surgery. Subclinical lymphedema was defined as asymptomatic lymphedema with an increase in L-Dex score of more than 10 units above baseline. Patients were stratified by lymph node dissection technique (i.e. sentinel SLNB or completion axillary ALND) and lymphedema treatment.
Patients were prospectively monitored preoperatively and assessed every 3 months within the first 2 years after surgery, in accordance with recommendations from Stout-Gergich [2008], unless recommended otherwise, such as in the case of positive assessment for lymphedema. At the first presentation of subclinical lymphedema, patients were treated using traditional methods (compression sleeve, massage, and/or physical therapy) as determined by the practicing physician. Resolution of BCRL was defined as a return to within 10 L-Dex units of the preoperative assessment at the end of follow-up.
Of the eligible patients, 210 underwent SLNB and 116 underwent ALND. L-Dex diagnosed lymphedema in 40 patients (12.3%); 9 (4.3%) who had undergone SLNB and 31 (26.7%) who had undergone ALND. Of those patients with subclinical lymphedema diagnosis, 50% of patients experienced resolution with treatment, 27.5% did not experience resolution despite treatment, and 22.5% of patients experienced resolution without treatment. Prevalence of persistent, clinical lymphedema was 0.5% for patients who had undergone SLNB and 8.6% for patients who had undergone ALND.
4. THE NATIONAL BURDEN OF LYMPHEDEMA
Although lymphedema has been receiving an increasing amount of scientific attention in recent years, the extent of its impact on the quality of patient's lives and its burden on society has not been appreciated.
The impact of lymphedema is felt immediately by the patient, who suffers from a debilitating swelling of an arm, leg, breast or torso, the pain that accompanies the swelling, impairment of physical activities, psychological and social problems, and frequent infections, often requiring emergency treatment. The burden on society is felt immediately by increased cost of treating preventable infections and long term by having to support the disabled patient who may no longer be able to make a living.
Treatment of lymphedema has been shown to reduce the incidence of recurrent cellulitis, and to retard the progression of lymphedema to harder-to-treat stages. It is therefore a very effective way to reduce the burden of lymphedema on the patient as well as the nation.
Incidence of Lymphedema [Weiss 2004-5]. Onset of lymphedema is shown to vary as a function of the method of measurement and the causative therapeutic procedure. Toxic effects of radiotherapy do not become fully evident until many years after treatment. Using sensitive lymphoscintigraphic measures of lymphedema, Campisi shows early effects of breast cancer treatment at 3-6 months (range <1 to 24 months). The delayed effects of radiotherapy are demonstrated by Pierquin in 1986 with median onset at 7 (range 2-37) months with surgery alone, 12 (1-52) months with surgery and radiation, and 25 (6-156) months with radiation alone [Campisi 2003]. Other researchers demonstrate medians between 1 and 2 years, with maximum times of onset of 3 to 10 years for mixed cohorts. Swelling after breast cancer treatment can occur at a number of body sites, and the restriction of measurements to one particular site such as the forearm, upper arm or entire arm and hand results in an underestimation of the incidence of lymphedema. Arm swelling may account for only about half of the patient-reported swelling [Bosompra 2002].
Other reported sites include the breast, chest, underarm and back. But measurement of these sites is very difficult, and so remains largely unreported. Breast lymphedema incidences of 70% using measurement of dermal swelling have been demonstrated [Rönkä 2004] while clinical examination detects only 35% in the same cohort. Changes in the mix of breast cancer surgery and radiotherapy over the last 50 years have resulted in a change in the incidence of lymphedema, since each therapy modality and combination has a different associated morbidity. Halsted radical mastectomies with and without radiotherapy, the standard until the 1970's, resulted in upper limb lymphedema rates of 22-44% without and with radiotherapy. With the ascendancy of the less radical modified radical mastectomy in the 1970's and 1980's lymphedema rates fell to 19-29% without and with radiotherapy [Schünemann 1997]. The 1990's brought breast- conserving surgery from a small percentage to approximately half of the surgeries performed [Yoshimoto 2004] with a further drop in upper limb lymphedema rates to 7-10% without and with radiotherapy [Schünemann 1997].
Breast lymphedema started to receive attention in 1982 with Kissin reporting clinical rates of 8% and Clarke reporting rates of 41% using skin measurements. Recent reports estimate the rates 1-9% based on subjective reporting [Fehlauer 2003][Højris 2000], 10-19% based on clinical examination [Fehlauer 2003][Goffman 2004], 20-48% [Rönkä 2004] [Senofsky 1991], and 30-70% based on skin thickness measurement [Rönkä 2004].
Lower limb lymphedema rates are likewise a strong function of the extent of the surgery and radiation used for treatment of reproductive and pelvic cancers, as well as lower limb melanomas. Whereas there are many different methods commonly used to evaluate upper limb swelling, there are very few methods reported to measure lower limb swelling. Lower limb lymphedema is reported in medical records only when it is severe enough that compression is not adequate, or causes disablement. Reported lower limb lymphedema ranges from zero [Coblenz 2002] to 60-80% [Balzer 1993][James 1982][Papachristou 1977] with many reports between these extremes.
Lymphedema of the genitals has been reported as 2-5% [Gaarenstroom 2003][Nelson 2004] and 18% (combined with lower limb) [Lieskovsky 1980]. Genital lymphedema among users of pneumatic pumps on the lower limb has been reported at 43% [Boris 1998].
Prevalence of Lymphedema. It is estimated that between 3 and 5 million patients in the United States suffer from lymphedema, with a significant proportion developing the disease as a consequence of cancer or its treatment. In oncology, the most common etiology for the development of lymphedema is the impaired or disrupted flow of lymph fluid through the draining lymphatic vessels and lymph nodes, usually as a consequence of surgery and/or radiation therapy. If the uninjured lymphatic vessels are unable to accommodate the increased lymphatic load, an accumulation of lymph fluid develops in the dependent tissues. Without intervention, lymphedema can lead to progressive swelling, fibrosis of the soft tissues, fat deposition, neurologic changes (e.g., pain and/or paresthesias), severe infection, chronic dermatitis, skin thickening and cracking. Heaviness of the affected limb induces difficulty in arm or leg use and causes shoulder or back pain [Hutchison 2010]. Lymphedema prevalence among cancer survivors increased from 0.95% in 2007 to 1.24% in 2013 [Brayton 2014].
Prevalence of primary lymphedema has been estimated as 1.15/100,000 persons under 20 years [Smeltzer 1985].
Population at Risk. Lymphedema is a medical condition affecting an estimated 1.5 to 3 million Medicare Beneficiaries who are not currently receiving treatment from Medicare according to the current medical standard of care. Medicare is spending billions of dollars every year treating largely preventable lymphedema-related cellulitis. Over time, untreated lymphedema results in disfigurement, disability and even death.
Cost of Not Treating Lymphedema – Case Study [Weiss 2007]. A number of separate approaches have been taken to arrive at a credible estimate of the potential savings to be achieved by treatment of lymphedema. One approach was to postulate two lymphedema treatment scenarios for a woman diagnosed with and treated for breast cancer. The first scenario postulates that she receives early and continued treatment of her lymphedema according to the recommended guidelines. The second scenario postulates that she receives no treatment for her lymphedema, but does receive medical treatment for her recurrent lymphedema-related infections. Data to support both scenarios are derived from statistics taken from recent scientific journals. The cost of lymphedema treatment over 40-year Breast Cancer Survivor's survival lifetime was $95,250 while the cost of medical treatment when the lymphedema was not treated was $340,000 which amounts to a saving of $244,750 over the 40 year lifetime of the illustrative patient (Cost Ratio = 3.57 no LE treatment / LE treatment).
Burden of Lymphedema [Shih 2009] A recent published study estimated the economic burden of breast cancer–related lymphedema (BCRL) among working-age women, the incidence of lymphedema, and associated risk factors. Claims data were used to study an incident cohort of breast cancer patients for the 2 years after the initiation of cancer treatment. Medical costs and rate of infections likely associated with lymphedema were compared between a woman with BCRL and a matched control. Approximately 10% of the 1,877 patients had claims indicating treatment of lymphedema. The matched cohort analysis demonstrated that the BCRL group had significantly higher medical costs ($14,877 to $23,167) and was twice as likely to have lymphangitis or cellulitis (OR= 2.02, P= .009). Outpatient care, especially mental health services, diagnostic imaging, and visits with moderate or high complexity, accounted for the majority of the difference.
Reducing the Burden with Early Intervention with Compression Garments [Stout 2008] In this prospective study, the authors demonstrated the effectiveness of a surveillance program that included preoperative limb volume measurement and interval postoperative follow-up to detect and treat sub-clinical LE. LE was identified in 43 of 196 (21.9%) women who participated in a prospective BC morbidity trial. Limb volume was measured preoperatively and at 3- month intervals after surgery. If an increase >3% in upper limb (UL) volume developed compared with the preoperative volume, then a diagnosis of LE was made, and a compression garment intervention was prescribed for 4 weeks. Upon reduction of LE, garment wear was continued only during strenuous activity, with symptoms of heaviness, or with visible swelling. Women returned to the 3-month interval surveillance pathway. Statistical analysis was a repeated-measures analysis of variance by time and limb (P < 0.001) comparing the LE cohort with an age-matched control group. The time to onset of LE averaged 6.9 months postoperatively. The mean (±standard deviation) affected limb volume increase was 83 mL (±119 mL; 6.5% ±9.9%) at LE onset (P = 0.005) compared with baseline. After the intervention, a statistically significant mean 48 mL (±103 mL; 4.1% ±8.8%) volume decrease was realized (P < 0.0001). The mean duration of the intervention was 4.4 weeks (±2.9 weeks). Volume reduction was maintained at an average follow-up of 4.8 months (±4.1 months) after the intervention.
Cost of a Lymphedema Treatment Mandate [Weiss 2016] The Commonwealth of Virginia has had a lymphedema treatment mandate since 2004. Reported data for 2004–2013, representing 80 % of the Virginia healthcare insurance market, contains claims and utilization data and claims-based estimates of the premium impact of its lymphedema mandate. The average actual annual lymphedema claim cost was $1.59 per individual contract and $3.24 per group contract for the years reported, representing 0.053 and 0.089 % of average total claims. The estimated premium impact ranged 0.00–0.64 % of total average premium for all mandated coverage contracts.
Ten years of insurance experience with a lymphedema treatment mandate in Virginia shows that costs of lymphedema treatment are an insignificant part of insured healthcare costs, and that treatment of lymphedema may reduce costs of office visits and hospitalizations due to lymphedema and lymphedema-related cellulitis. Estimates based on more limited data overestimate these costs. Lymphedema treatment is a potent tool for reduction in healthcare costs while improving the quality of care for cancer survivors and others suffering with this chronic progressive condition.
5. Medicare COVERAGE of these Items
Protocols for Lymphedema Treatment The recognized standard of treatment of lymphedema is Complete/Complex Decongestive Therapy (CDT). CDT comprises four interacting protocols applied as required for the individual patient in two phases (acute and ongoing): manual lymph drainage (MLD); compression therapy; lymph drainage exercises; and skin care [ACS 1998, ISL 2013, NLN 2011]. A recent technology assessment has determined that there is moderate to good evidence that these protocols, with or without adjuvant sequential pneumatic compression, will result in an improvement in the health of lymphedema patients [MEDCAC 2009]. The initial intensive phase is performed by specially-trained medical professionals, but ongoing care is patient self-provided at home using techniques taught the patient by the healthcare provider.
Treatment procedures have been developed in Europe over the last 50 years, and have been accepted by American medicine for the last 20 years.
(1) The current standard of treatment for lymphedema is called complex (or complete) decongestive therapy (CDT) and has been the recommended lymphedema treatment protocol in Europe since 1995 (International Society of Lymphology) and in the U.S. since 1998 [ACS 1998]. CDT comprises a number of interrelated treatment modalities that are most efficacious when utilized in an interdependent fashion. In a meeting on November 18, 2009 the Medicare Evidence Development Coverage Advisory Committee (MEDCAC) affirmed that there is adequate evidence available to support CDT, with or without adjuvant pneumatic sequential compression, as being beneficial in the treatment of lymphedema. [MEDCAC 2009]
(2) Complex decongestive therapy for the acute treatment program (Phase 1) consists of manual lymph drainage, short-stretch bandaging, remedial exercise, skin care, and instruction on self massage and home maintenance, including the fitting and procurement of compression garments, bandages and devices. Pneumatic sequential compression may be beneficial as an adjunct to CDT but should never be used in its absence. Treatment duration and frequency for the acute treatment program (Phase 1) as well as follow-up treatments shall be dictated by medical necessity, as determined by a licensed physician knowledgeable in diagnosis and current management standards of the treatment of lymphedema.
(3) After effective volume reduction has been accomplished through the combined effects of these modalities, Phase 2 of the treatment program, the home treatment or maintenance phase, continues for the life of the patient according to an individual medical treatment plan, and comprises, as directed by a licensed physician knowledgeable in diagnosis and current management standards of the treatment of lymphedema: self-manual lymph drainage; day-time use of well-fitted compressive garments, night-time bandaging with multi-layer low-stretch bandages or manually-adjustable compression devices; skin care; and exercise while under compression. It is generally not helpful to fit compressive garments prior to the institution of volume-reducing techniques. The compressive garment should be fitted to apply an appropriate range of external pressure, generally between 20 and 60 mmHg. It is recommended that garments be replaced every 3-6 months to maintain maximal therapeutic benefit.
(4) There are numerous accredited schools that teach the CDT protocols for lymphedema treatment to licensed physical therapists, occupational therapists, massage therapists and nurse practitioners. The Lymphology Association of North America (LANA) provides national certification of lymphedema therapists who meet stringent academic and experiential requirements and who are qualified to perform the medical protocols involved in lymphedema treatment
What Does Medicare Cover? Medicare covers and pays for rehabilitative therapy by Physical Therapists and Occupational Therapists that can include manual lymph drainage (although Medicare does not require that the provider of lymphedema treatment services be qualified in the specialized lymphedema treatment techniques). Application of compression bandage systems after MLD is bundled with the manual therapy, but the cost of the bandages is not covered. Since the compression garments that must be worn at home on a daily basis are not covered, measurement and fitting costs are not covered. A limited number of instructional sessions on the home management of lymphedema are covered. Medicare also covers pneumatic sequential compression pumps after a failed trial period of conservative treatment and a failed trial with a simpler venous pump, and a limited number of training sessions to instruct the patient on home use of the pumps.
Medicare does not currently cover lymphedema treatment materials (compression bandages, garments, devices or supplies) that are the mainstay of chronic lymphedema treatment and management in a home setting. Many patients still suffer recurrent infections, progressive degradation in their condition and eventual disability because they cannot afford the compression bandages and garments required for their everyday self-care.
Over the last decade, until 2006, Medicare listed the individual components of the bandaging systems as “primary surgical dressings” (HCPCS Code Axxxx), and compression stockings and sleeves as “prosthetic services” (HCPCS Code Lxxxx). Insurers, recognizing that the code L8010 Breast prosthesis, mastectomy sleeve was a “breast prosthesis” code and not appropriate for lymphedema without a breast prosthesis, had a special group of codes added to the HCPCS Code Book under Prosthetic Services, Gradient Pressure Aid for compression sleeves and gloves (S8420-S8428) to provide a national standard description. A special set of codes was added (S8429-S8431) for the short-stretch bandages and padding that made up a lymphedema bandaging system to differentiate from the A-Codes of surgical dressings. The S-Codes were not reimbursed by Medicare, but served as uniform national descriptors for insurance reimbursement. For treatment of lower limb lymphedema prosthetic service Elastic Supports codes L8100-L8239 were used since these were already prosthetic services codes. [HCPCS 2003]
In March 2006 CMS re-coded the compression stockings that are the mainstay of lower limb lymphedema treatment from "prosthetic services" (the benefit category covering compression garments for lymphedema) to "secondary surgical dressings" (a benefit category which is not covered in a home setting or without presence of an open wound). This administrative change effectively denied millions of lymphedema Medicare beneficiaries coverage of the medical treatment which they require every day for the rest of their lives. The change was made as an administrative change without prior notice and without any chance for public or expert testimony on the medical impact of the change. There was never any justification, analysis or impact statement published [See Appendix A].
Denial rests on an erroneous CMS interpretation of title XVIII of the Social Security Act (SSA) that looks at the "form" of the item and not its "function" in determining the benefit category despite the fact that the SSA defines benefits in terms of medical function. The result of this administrative change in HCPCS Code of the medical items used in the treatment of lymphedema was to completely eliminate them as prosthetic services, which they ARE when one looks at their medical FUNCTION in the treatment of lymphedema, and to define them as secondary dressings which are not covered in a home setting and which must be used to secure a primary dressing in the treatment of an open wound. Although HCPCS Codes explicitly cannot confer or deny coverage status, once an A-Code was assigned, the coverage criteria for surgical dressings in the LCD for Surgical Dressings was used to deny them coverage.
Statutes and Policies Regarding Prosthetic Devices. The Medicare Benefit Coverage Manual, CMS Publication 100-02, Chapter 15, §120 defines "Prosthetic devices (other than dental) which replace all or part of an internal body organ (including contiguous tissue), or replace all or part of the function of a permanently inoperative or malfunctioning internal body organ are covered when furnished on a physician's order." The lymphatic system constitutes an "internal body organ system" and compression therapy replaces part of the function of this malfunctioning internal body organ.
I have been assisting Medicare beneficiaries in appeals of their denials of lymphedema treatment materials. My legal and medical arguments, supported by references to medical sources as well as relevant Medicare laws and policies, make the case that compression therapy materials (specifically: compression bandaging systems; compression garments; directional flow pads and garments; non-elastic compression devices) are reasonable and medically necessary in the treatment of lymphedema according to current medical standards [Appendix B].
Furthermore, they meet all of the statutory requirements of prosthetic devices according to Section 1861(s)(8) of title XVIII of the SSA as interpreted by HCFA and CMS in the Medicare Benefit Policy Manual, CMS Publication 100-02, Chapter 15, §120 “Prosthetic Devices”, and therefore cannot be denied on the basis "they do not fit any statutory benefit category."
On August 16, 2006 a U.S. Administrative Law Judge in Southern California found that certain items received by a Medicare beneficiary for the treatment of lymphedema "were reasonable and necessary for reimbursement as a prosthetic device" and that "payment is to be made on the beneficiary's behalf for the reasonable and necessary medical services rendered and billed." The items included compression bandaging materials, a directional flow sleeve, compression arm sleeve and gauntlets. Visits to a lymphedema treatment center for the purposes of evaluation and measurement and fitting of the sleeves and gauntlets were also allowed.
This decision was applicable only to the single case heard, and did not change Medicare policy or set precedent in other cases. But it was encouraging in that the initial decision of this Judge was based on principles of law and on current medical knowledge of the treatment of lymphedema, and gives hope that we can achieve an upgrade to Medicare coverage of lymphedema treatment without any legislative change in the law, but with new policies based on a fresh interpretation of the existing law [Weiss 2006].
Since that initial ALJ determination dozens of U.S. Administrative Law Judges have confirmed the fact that compression bandages, garments and devices, when used in the compression therapy of lymphedema, meet the statutory definition of “prosthetic devices” and are covered by Medicare.
6. DESCRIPTION OF THE ITEMS OF SERVICE
The Science Behind Compression Therapy in Lymphedema Management [Zuther Dec 5, 2014] It is important to understand that the elastic fibers in the tissues affected by lymphedema are damaged. These fibers lose their elasticity and tend to harden, which is particularly the case in untreated lymphedema present over a long period of time and progressed stages of lymphedema. Although the swelling in lymphedema may be reduced to a normal or near normal size during treatments, the damage to the lymphatic system, which caused the onset of lymphedema, is permanent and the skin elasticity in the tissues affected by lymphedema may never be regained to prior levels.
Different from acute edema, which is a low-protein swelling, lymphedema is a disease rather than a symptom, and its underlying cause, the insufficiency of parts of the lymphatic system, cannot be reversed. Lymphedema results from the inability of the lymphatic system to perform one of its basic functions, the removal of water and protein from the tissues of a part of the body. This insufficiency can be caused by developmental abnormalities of the lymphatic system (primary lymphedema), or damage to the lymphatic system such as the removal or radiation of lymph nodes in cancer surgery, scarring or infection of the lymphatic system, damage to or fibrosing of the lymphatic valves or filamentary suspension, or damage to the autonomous neurological system (secondary lymphedema).
Compression garments for lymphedema function as prosthetic devices, replacing all or part of the function of a permanently inoperative or malfunctioning internal body organ. The prescribed compression compensates for the loss of function of the lymphatic collectors and/or lymphangion pump. Since lymph vessels have their own intrinsic contractions, removal or damage to the lymph vessels or their surrounding support tissue requires an outside source of pressure and excitation to move the lymph that will otherwise stagnate and lead to the adverse medical consequences described above [Hutchison 2010].
The accumulation of protein and water in the tissues may be gradual in some patients and sudden in others. Lymphedema does not dissipate by itself and continues to progress without adequate treatment.
The goal of lymphedema management is to reduce the lymphedematous swelling to a normal or near normal size utilizing remaining healthy lymph vessels and alternative lymphatic pathways. Once the lymphedema is decongested, the secondary goal is to maintain the reduction and to prevent the re-accumulation of tissue fluid. These goals can be achieved with the internationally recognized “gold standard” of lymphedema treatment known as complete or complex decongestive therapy (CDT). CDT is a combination of the treatment modalities: manual lymph drainage (MLD); skin care; decongestive exercises; and compression therapy.
Compression therapy in lymphedema management is provided either via bandages, compression garments or alternative compression devices, the selection of which depends on the stage of treatment. Compression bandages and garments by themselves will not reduce existing swelling [Rogan 2016] and must therefore not be worn on an untreated, swollen extremity.
Individuals affected by lymphedema graduate from padded short-stretch bandages, which are applied by the lymphedema therapist in the intensive phase of CDT, to elastic or non-elastic compression garments when the affected extremity is decongested. To assist in the movement of fluids back to the heart, a pressure gradient between the lower (higher pressure) and the upper part (lower pressure) of the extremity is provided with bandages and garments.
Even after successful treatment, the body part affected by lymphedema is at permanent risk for re-accumulation of fluid and most individuals affected by lymphedema are aware of the fact that this condition requires life-long care. Without the benefits of external compression successful long-term management of lymphedema would be very difficult and in most cases impossible.
Indications for Compression Bandaging [Chikly 2001] One of the first effects of chronic lymphedema is the destruction of the elastic fibers of the conjunctive tissue, a state called "elastic insufficiency." Consequently, without bandaging, the skin is likely to remain stretched, and stagnant fluid may re-accumulate soon after evacuation by the practitioner. Most significant lymphedemas need careful bandaging to maintain the gain of the hands-on techniques. Remember that the hands-on modalities are only applied once or twice a day for 1 to 2 hours. What is stimulating the lymph to flow during the remaining 21 to 23 hours of the patient's day? The external compression helps stimulate lymph flow and helps prevent the tissue from filling up.
At first, the pressure of the edema decreases more than its volume.
•The skin has high elasticity and low resistance. Over time, in response to stretching pressure from the edema, it loses some of its elasticity. The bandage is a substitute for the skin's external pressure; it is like an additional fascia layer.
•The skin may not return immediately to its normal tension. This stretching-out and slow pulling back to normal is called the hysteresis ("creeping" or "lagging behind") effect.
Compression therapy is an essential component in lymphedema management As explained, in lymphedema the elastic fibers in the extracellular spaces are damaged and thus unable to provide sufficient resistance to the build-up of fluid in the interstitial tissue spaces. The application of external compression provides the necessary support for those tissues that lost elasticity and compensates for the elastic insufficiency by increasing the tissue pressure. The tissue pressure plays an essential role in the exchange of fluids between the blood capillaries and the tissue. The increased tissue pressure provided by external compression reduces the amount of fluid leaving the blood capillaries into the tissues and increases the return of tissue fluids into the lymph collectors and capillaries, thus reducing the amount of fluid in the tissue.
External compression also increases venous and lymphatic return by improving the function of the valves in these vessels. Another important factor for sufficient return of venous and lymphatic fluids back into the blood stream is the movement of skeletal musculature and joints during activity. Together with other supporting mechanisms the muscle and joint pump activity propels these fluids back to the heart and ensures uninterrupted circulation. External compression provides a counter force to the working musculature, known as working pressure, thus improving its efficiency.
These effects help to prevent re-accumulation of fluids that were evacuated during intensive CDT treatments and conserve the results achieved during MLD.
Another positive impact of compression therapy is the softening of hardened connective tissue often present in lymphedema, especially if external bandaging is combined with special foam materials. Some compression sleeves and stockings are made with a textured material designed to stimulate and direct lymphatic flow.
The essential nature of continuing patient adherence to the program of CDT including regular compression was recently shown in a five-year study that demonstrated that the group of adherent post-mastectomy lymphedema patients maintained the limb reduction achieved in the intensive phase of CDT and scored higher than a group of non-adherent patients in a quality of life evaluation whose arms grew by 14%. [Ochalek 2014].
Forms of Compression Items In his on-line blog, lymphedema expert Joachim Zuther, co-author of Lymphedema Management—The Comprehensive Guide for Practitioners [Zuther 2005] expands on the major types of compression items and how they are used.
(1) Compression Bandage Systems [Zuther Jan 12, 2012] Compression therapy, like manual lymph drainage (MLD), exercises and skin care, is a main element of Complete Decongestive Therapy (CDT). In most cases, the elastic fibers in skin tissues affected by lymphedema are damaged and unable to provide adequate resistance against the musculature working underneath, and the blood and lymph vessels within these tissues. External compression compensates for the elastic insufficiency of the affected tissue, providing the resistance necessary to maintain the reduction of the swelling and to prevent re-accumulation of tissue fluid.
Compression bandages are used during the intensive phase of CDT. In this sequence of the treatment the volume of the affected limb changes almost on a daily basis, and it is necessary that external compression adapts to these changes. Bandages are much better suited for this task than compression garments (sleeves, stockings), which would have to be re-fitted constantly. Garments are used in the second phase of CDT, when the limb is decongested and volume changes are minimal.
Crucial in lymphedema management is to provide the skin tissues with a solid counterforce against the muscles working underneath, particularly while standing, sitting, walking, or performing therapeutic exercises. The subsequent increase in the tissue pressure during muscle activity promotes lymphatic and venous return, and prevents fluid from accumulating in the skin. It is equally important to prevent the bandages from exerting too much pressure on the tissues during rest, which could cause a tourniquet effect and effectively prevent adequate return of these fluids.
There are two distinct types of compression bandages – short-stretch and long-stretch bandages. The difference refers to the extent the bandages can be stretched from their original length. Short-stretch bandages are made from cotton fibers, which are interwoven in a way that allows for about 60% extensibility of its original length, whereas long-stretch bandages, commonly known as “Ace” bandages contain polyurethane, which allows for an extensibility of more than 140% of the bandage’s original length.
The extent of which a bandage can be stretched specifies the two main qualities of pressure in compression therapy – the working pressure and the resting pressure. The working pressure is determined by the resistance the bandage provides against the working musculature underneath, and is active only during muscle activity, and therefore temporary. The pressure the bandage exerts on the tissues at rest, i.e. without muscle contraction, is known as the resting pressure, which is permanent. Relevant to these pressure qualities are the number of bandage layers, the tension with which these layers are applied, and most importantly the type of bandage used.
The high working pressure of short-stretch cotton bandages provide the necessary solid counterforce and make them the preferred compression bandage in the management of lymphedema. Due to the low resting pressure of short-stretch bandages, tourniquet effects are prevented – provided these bandages are applied correctly.
Long-stretch “Ace” bandages have the exact opposite effect and are not suitable for lymphedema management. The low working pressure these bandages provide does not offer adequate resistance, and fluid would inevitably accumulate. In addition, the high resting pressure of long-stretch bandages could constrict veins and lymph vessels during rest.
“Compressive bandages, when applied incorrectly, can be harmful and/or useless. Accordingly, such multilayer wrapping should be carried out only by professionally trained personnel.” [ISL 2013]
Compression bandages must not have a constrictive effect or prevent movement in the joints to too great a degree. The patient must be able to carry out exercises with the bandage on, to improve lymphatic transport capacity [Schingale 1996].
Applying the compression bandage [Schingale 1996]
- After the necessary skin care with acid-buffered ointment, a tubular bandage is applied, the total length of which is slightly greater than that of the extremity in question. This tubular bandage prevents direct contact of the padding with the skin, to avoid allergic reactions. In addition, contact is avoided between the ointment and the padded dressings and perspiration is absorbed.
- When applying a finger bandage, the fingertips must be left free, so that compression of the arterial vessels can be monitored. If the fingertips are livid after exercises, the bandage is too tight. To avoid impairment of movement, the fingers must be spread during bandaging. Once the bandage is in place, the patient must still be able to make a fist.
Any further heavy duty bandages must be applied so that pressure is highest distally and lowest proximally, allowing lymph flow towards the centre.
- Additional increases in pressure are achieved not only by firm application of the bandage, but also by overlapping individual layers of bandages several times. This technique may be used to increase the distal to proximal pressure gradient.
- Where oedema of the leg is associated with oedema of the corresponding quadrant of the trunk, the body may be bandaged up to the hip; for the arm, the chest may also be bandaged.
- If bandages are applied in stages, rebandaging in the event of loosening of the thigh area is easier while the lower bandages remain firmly in place, since they are held with strips of adhesive tape. Where the upper thigh is particularly wedge-shaped, support may be improved by applying foam rubber pads in the upper region [Schingale 1996].
Standard Layers For Lymphedema Bandaging; Follow-Up Guidelines [Chikly 2001]
1. Water-based, non-perfumed lotion that will not degrade the elastic garment, it has a low pH and is lanolin based. Keep the skin moist, supple, pliable and well-lubricated, e.g., EucerIn® Lotion.
2. Finger or toe gauze bandages (optional): These can reduce swelling induced by the bandage pressure proximal to them.
3. Soft cotton stockinette for contact with the skin; this protects the bandage and the skin against salt deposition from perspiration, and creates a buffer between the skin and the synthetic material. Stockinette is quite 14 stretchy" and exerts little elastic tension. A double layer may be necessary if the patient has a reaction to the padding.
4. Synthetic padding, e.g. Artiflex®; this helps in breaking down fibrosis, preventing indentation from bandaging, and protecting bony parts and body concavities. Cotton is not suitable here; cotton can absorb water, harden and press too hard in spots.
5. Low-stretch, low-resting pressure, high-working pressure bandages: Comprilan®, Rosidal® or Dema-Band®.
(2) Compression Garments [Zuther Sep 19, 2010] The external support provided by compression garments are an essential component of lymphedema management. Without the benefits of compression therapy, the lymphatic fluid removed by successful treatments would re-accumulate, and long-term management of lymphedema would be impossible.
The use of elastic stockings is considered a valuable component of lymphedema therapy, and appears to be critical to the long-term success of treatment. Compliance with elastic stocking may be problematic since they are frequently hot, uncomfortable, and considered unsightly by some. Lack of compliance may result in requests for further treatment, such as pneumatic pumps or complex decongestive physiotherapy. However, elastic garments are a component of all treatments of lymphedema and compliance has a major impact on the success of any treatment of lymphedema. [Aetna 2015]
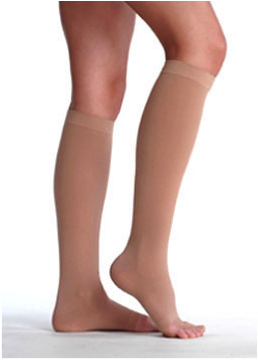
Compression garments for extremities such as sleeves, gauntlets, stockings and pantyhose, or those manufactured for other parts of the body (vests, brassieres) are available in several sizes, variations and compression classes.
The level of compression within the different classes is determined by the value of pressure the garments produce on the skin; these pressure values are measured in units of millimeters of mercury (mmHg). For a compression garment to work effectively, the pressure needs to gradually decrease from the most distant part of an extremity (ankle, wrist) to the nearest part (shoulder, hip). This gradient is necessary to avoid tourniquet effects and subsequent obstruction of lymph flow.
Most manufacturers in the United States use the following pressure values within the compression classes:
- Compression Class 1: 20-30 mmHg
- Compression Class 2: 30-40 mmHg
- Compression Class 3: 40-50 mmHg
- Compression Class 4: over 50 mmHg
In general, compression levels provided by class 2 garments will be sufficient to prevent swelling in most patients affected by lymphedema of the upper extremity; patients with involvement of the leg will usually require a garment of compression class 3.
However, there are a number of exceptions to this general rule. Some patients with lower extremity lymphedema may require garments of lower compression levels than those provided in class 3, or maybe a garment of a higher compression. Alternatively, patients with lymphedema of the arm may use a sleeve of compression class 1, or even class 3 in some cases.
Many factors must be considered by the physician and/or lymphedema therapist in order to determine the correct compression class for each individual patient. Tolerance to external compression, age, activity level, skin integrity and possible additional conditions, such as arterial insufficiencies or heart problems may influence the level of compression.
Compression garments for the treatment of lymphoedema maintain the best possible level of decongestion with manual lymph drainage, compression bandaging and specific exercises, but must in all cases be made to measure. The severity and location of the oedema, the age of the patient and the presence of any secondary illnesses are decisive for the category of compression and the type of care given [Schingale 1996].
Care of lymphoedema of the arm: Normally compression class II, since these sleeves can be applied by the patient without assistance. Patients with secondary diseases such as pain, rheumatic disease or advanced age are an exception. In these cases, compression class I may be prescribed, if necessary with two sleeves worn, one on top of the other. Where the back of the hand and/or fingers are oedematous, a compression glove is required. If there is pronounced proximal oedema, sleeves with a shoulder cap and support are prescribed. given [Schingale 1996].
Care of oedema in the lower extremity: Normally compression class III and in younger patients also class IV. In the event of pronounced lymphoedema of stages II-III, it may be necessary to wear a further class II or III stocking in the lower leg area. If the oedema and fibrosis is more pronounced in the area of the ankles, short socks with ankle pads are prescribed. For oedema of the toes, compression toe caps are necessary. Compression tights are normally prescribed in these cases, or stockings if only one extremity is affected. The use of Bermuda tights over the normal tights is successful in some cases, but care must be taken to ensure that compression decreases from the distal to the proximal area [Schingale 1996].
Compression garments are part of the daily life of patients with lymphoedema. Patient-friendly compression garments make daily compression therapy easier, improve compliance and therefore contribute to the success of treatment. Continuous research and development have led to the manufacture of medical compression garments for use in lymphoedema. Compression garments for use in lymphoedema are made from special materials on computer-controlled machines. High permeability to air and elasticity suitable for the particular condition, guarantee the best wear characteristics.
Medical garments for the compression treatment of lymphoedema are available in the form of gloves, sleeves and stockings. They come in various sizes and compression classes. They are made individually and as unique items, to the specification of the prescribing doctor and in accordance with the measurements taken.


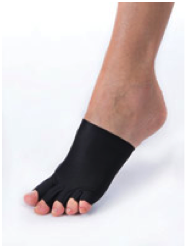
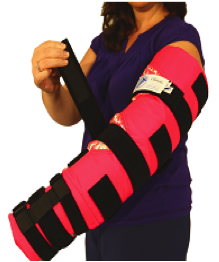
(3) Strapped Adjustable Garments or Devices, nonelastic arm or leg binders (e.g., CircAid, LegAssist, Reid Sleeve) are considered medically necessary for persons with lymphedema who meet the selection criteria for pressure gradient support [sleeves or] stockings [Aetna 2014]. Nonelastic [arm or] leg binders are similar to graded compression [sleeves or] stockings in that they provide static compression of the [arm or] leg, but unlike graded compression [sleeves or] stockings, they do not use elastic, but use non-elastic fabric and adjustable Velcro or buckle straps.
Typical adjustable compression devices designed to emulate the compression created by a compression bandaging system are the CircAid and the FarrowWrap. The key to good maintenance is adequate graduated compression, which not only requires patient compliance, but also the availability of suitable garments for the affected limb. Although the range of compression garments and applicators has improved in recent years, there are still circumstances where they are not suitable for certain groups of patients. These include patients with weak hand strength, back problems, obesity, abnormal limb shape, and elderly or palliative patients [Lawrance 2008].
Farrow Wrap™ has been designed to address the needs of this group of patients. This system uses the principles of short-stretch bandaging, providing a low resting/high working pressure garment. Farrow Wrap™ consists of a protective silver liner, over which the wrap is applied — multiple overlapping short-stretch bands interconnected by a spine. The bands are secured using Velcro®, and the degree of compression is determined by the user applying the wrap at near end stretch of the material, as well as by the circumference of the leg and the position and activity of the user. Graduated compression is achieved by the end stretch of the material, as well as the shape of the limb in accordance with the modified La Place’s law. The system is flexible, however, allowing the user to vary the degree of compression to the limb, depending on patient requirements. The Farrow Wrap™ system consists of a foot wrap, a lower leg piece, and the option of a thigh wrap, which interconnects to the below-knee garment with Velcro®. [Lawrance 2008].

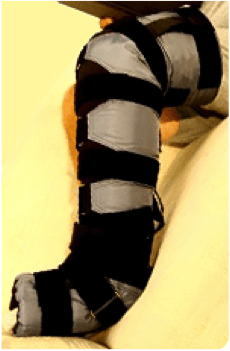
A typical adjustable compression device incorporating the directional flow feature is the Reid Sleeve, manufactured by Peninsula Medical, Inc. [Peninsula 2015]. The ReidSleeve (U.S.Patents: 5,904,145; 5,916,183; 5,196,231) is made from a soft foam core. This is specially designed to provide a gentle gradient pressure. The pressure exerted on the limb (arm or leg) is controlled by a series of Velcro® straps. This design allows the compression to be precisely tailored to the patient’s needs. The sleeve easily slides over the affected limb and then the compression straps are adjusted.
The outer shell is made of nylon, and the inner lining is made of a blend of cotton and lycra to provide maximum comfort while maintaining effectiveness.
Control of edema in the hand and wrist area is critical. The ReidSleeve incorporates conforming plastic plates to provide consistent, effective pressure to this critical area. The data from the original study demonstrates that the ReidSleeve is highly effective at controlling edema in this area. In a 4-week study, edema in the hands of affected patients was reduced by an average of 80%.
A specially designed gauge (U.S. Patent 5,904,145) is used to assess the pressure exerted over any region of the limb. The gauge is as easy to use as a blood pressure cuff. This simple procedure insures that compression applied to the patient's limb is consistently applied and in the proper range to provide optimal results.
Patients can fit the sleeve in minutes without assistance and have the confidence of knowing they are applying the most effective pressure. As the patient improves the ReidSleeve can be adjusted to the new limb size thereby maintaining the proper pressure range.
(4) Directional Flow Garments and Pads “Compressive bandages, when applied incorrectly, can be harmful and/or useless. Accordingly, multilayer wrapping should be carried out only by professionally trained personnel. Newer manufactured devices (i.e. pull-on, Velcro-assisted, quilted, etc.) may relieve some patients of the bandaging burden and perhaps facilitate compliance with the full treatment program…” [ISL 2009]
Directional flow garments provide gradient compression, and are custom-manufactured for each patient to provide Class 2, 20-30mmHg of compression. An optional elastic outer jacket adds approximately 10mmHg when worn together with the garment to provide Class 3, 30-40mmHg. The efficacy of custom directional flow garments, such as the Tribute Night™ is due only in part to their compression values. Garment construction features quilted, bi-directional channels filled with medical grade foam particles of varying sizes, shapes and densities. This creates a localized tissue stretch and skin surface pressure differential, stimulating lymphatics, gently breaking down fibrosis, and guiding fluid and cellular waste toward collateral pathways.

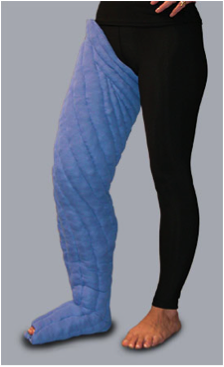
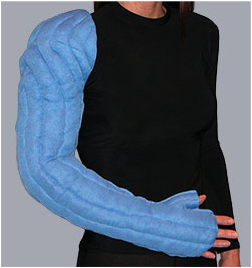
Directional flow garments may be a safer and more manageable alternative to the multi-layer compression bandaging protocol which is the clinical standard for the intensive phase of complex decongestive therapy and an integral part of the home management phase of treatment. They free the user from the necessity of mastering the application of multi-layer compression bandaging systems, and provide a safe, easy and effective alternative. They allow the user to sleep in comfort, in their natural positioning, receiving effective, therapeutic treatment while they sleep. [Adapted from Tribute 2015]
Intensive Treatment in a Clinical Setting An essential part of the clinical phase of lymphedema treatment (Phase 1, Intensive Phase) is the application of a system of short stretch bandages to maintain the edema reduction achieved by manual lymph drainage. The bandage system, comprising finger or toe wraps, tubular sleeve, foam forms, foam or non-woven padding, and short-stretch bandages, is applied by a therapist who is specially trained and certified in the techniques of lymphedema management. Although the application of these materials is usually bundled with the lymphatic drainage that directly precedes it (i.e. CDT 97140), the cost of these materials is not covered by Medicare.
Each element of a lymphedema bandage system has a unique medical function, and all must be used in a single application to the lymphedema patient. Although they resemble materials commonly used as disposable surgical dressings, their specifications and their medical use in the treatment of lymphedema is different. They are each an element of a custom compression garment that is applied each day, required to contain the swelling that exists at that time. They are often sold as kits, since the same materials in the same combination are required by many different patients. These bandages are reused each day after washing and re-rolling, and are not discarded after every use.
The Intensive Phase is continued until the limb or body site swelling has been reduced to a plateau, at which time the patient is measured for a compression garment which is worn to maintain this reduced state and prevent further swelling of the lymphedematous site. Elastic garments are usually indicated for daytime use, but custom flat-knit garments may be required because of the patient’s special medical needs. Night-time compression may require the wearing of a non-elastic night garment or device. Measurement for a maintenance garment is done by a therapist or supplier who is trained and certified to perform this service by the manufacturer of the prescribed garment. It is important that the garment fit properly.
Here again, Medicare does not cover these compression garments, nor for measurement and fitting of the garments.
There is a misconception by some insurers about the distinction between off the shelf (OTS) and custom compression garments. OTS sleeves and stockings are manufactured by a “circular knit” process which produces a seamless, elastic compression garment. This type of garment is useful for treatment of early stages of lymphedema where the limb is regular and generally tapered in geometry. In more severe cases, or when the limb is not regularly shaped, has locally massive swelling, or is outside the normal sizes, a different type of garment is needed. These custom garments are manufactured using a “flat knit” process and result in a seamed, stiff garment fitted to the irregular geometry of the patient’s limb. The custom garment provides medical treatment unavailable in the OTS garment. The difference between the off-the-shelf and custom garments is not just price.
Maintenance Treatment may be covered if skilled service is required While an expectation of improvement would be a reasonable criterion to consider when evaluating, for example, a claim in which the goal of treatment is restoring a prior capability, Medicare policy has long recognized that there may also be specific instances where no improvement is expected but skilled care is, nevertheless, required in order to prevent or slow deterioration and maintain a beneficiary at the maximum practicable level of function. [CMS 2013] Specifically, in accordance with the Jimmo VS Sebelius settlement agreement, manual revisions will clarify that coverage of therapy "...does not turn on the presence or absence of a beneficiary's potential for improvement from the therapy, but rather on the beneficiary's need for skilled care." [CMS 2013]
7. BENEFIT CATEGORY INTO WHICH LYMPHEDEMA COMPRESSION ITEMS FALL
Requirements for Medicare Coverage “Medicare payment is contingent upon a determination that: 1) A service meets a benefit category; 2) Is not specifically excluded from coverage; and 3) The item or service is “reasonable and necessary.” [CMS 100-16] In terms of the provisions of the Social Security Act, this means:
1) It is one of the benefit categories described in Title XVIII of the Social Security Act (the Act) e.g. §1861 Definitions of Services;
2) It is not excluded by Title XVIII of the Act, other than Section 1862(a)(1) Exclusions from Coverage, and;
3) It is reasonable and necessary under Section 1862(a)(1) of the Act”
[DME MAC A Supplier Manual]
Defined Benefit Categories DMEPOS Categories in Title XVIII: There are four benefit categories defined in §1861 that are relevant to this discussion. These are the four benefits usually combined into the broad category of Durable Medical Equipment, Prosthetics, Orthotics, and Supplies (DMEPOS) for administrative convenience. These separate covered categories are defined in §1861(s) Medical and Other Health Services in sub sections:
(5) surgical dressings and splints, casts, and other devices used for reduction of fractures and dislocations [supplies];
(6) durable medical equipment (also defined in §1861(n);
(8) prosthetic devices (other than dental) which replace all or part of an internal body organ (including colostomy bags and supplies directly related to colostomy care), including replacement of such devices, and including one pair of conventional eyeglasses or contact lenses furnished subsequent to each cataract surgery with insertion of an intraocular lens;
(9) leg, arm, back, and neck braces, and artificial legs, arms, and eyes, including replacements if required because of a change in the patient’s physical condition; [prosthetics and orthotics]
DMEPOS Categories in the Medicare Benefit Policy Manual: The four benefit categories included within the definition of DMEPOS are further described in Chapter 15, §§100-130 of the Medicare Benefit Policy Manual (MBPM), CMS Publication 100-02. Abstracts from those sections follow:
§100 – Surgical Dressings, Splints, Casts, and Other Devices used for Reductions of Fractures and Dislocations. Surgical dressings are limited to primary and secondary dressings required for the treatment of a wound caused by, or treated by, a surgical procedure that has been performed by a physician or other health care professional to the extent permissible under State law. In addition, surgical dressings required after debridement of a wound are also covered, irrespective of the type of debridement, as long as the debridement was reasonable and necessary and was performed by a health care professional acting within the scope of his/her legal authority when performing this function. Surgical dressings are covered for as long as they are medically necessary.
§110 – Durable Medical Equipment. Durable medical equipment is equipment which:
• Can withstand repeated use;
• Is primarily and customarily used to serve a medical purpose;
• Generally is not useful to a person in the absence of an illness or injury; and
• Is appropriate for use in the home.
All requirements of the definition must be met before an item can be considered to be durable medical equipment. Expenses incurred by a beneficiary for the rental or purchases of durable medical equipment (DME) are reimbursable if the following three requirements are met:
• The equipment meets the definition of DME (§110.1);
• The equipment is necessary and reasonable for the treatment of the patient’s illness or injury or to improve the functioning of his or her malformed body member (§110.1); and
• The equipment is used in the patient’s home.
§120 Prosthetic Devices. Prosthetic devices (other than dental) which replace all or part of an internal body organ (including contiguous tissue), or replace all or part of the function of a permanently inoperative or malfunctioning internal body organ are covered when furnished on a physician’s order.
Examples of prosthetic devices include artificial limbs, parenteral and enteral (PEN) nutrition, cardiac pacemakers, prosthetic lenses (see subsection B), breast prostheses (including a surgical brassiere) for post-mastectomy patients, maxillofacial devices, and devices which replace all or part of the ear or nose. A urinary collection and retention system with or without a tube is a prosthetic device replacing bladder function in case of permanent urinary incontinence. The Foley catheter is also considered a prosthetic device when ordered for a patient with permanent urinary incontinence.
The coverage of prosthetic devices includes replacement of and repairs to such devices as explained in subsection D.
§130 – Leg, Arm, Back, and Neck Braces, Trusses, and Artificial Legs, Arms, and Eyes. These appliances are covered under Part B when furnished incident to physicians’ services or on a physician’s order. A brace includes rigid and semi-rigid devices which are used for the purpose of supporting a weak or deformed body member or restricting or eliminating motion in a diseased or injured part of the body. Elastic stockings, garter belts, and similar devices do not come within the scope of the definition of a brace. Back braces include, but are not limited to, special corsets, e.g., sacroiliac, sacro-lumbar, dorso-lumbar corsets, and belts. A terminal device (e.g., hand or hook) is covered under this provision whether an artificial limb is required by the patient. Stump stockings and harnesses (including replacements) are also covered when these appliances are essential to the effective use of the artificial limb. Adjustments to an artificial limb or other appliance required by wear or by a change in the patient’s condition are covered when ordered by a physician.
Flow Down of Statutory Requirements Traceability of requirements is maintained throughout all of the Federal Regulations and the Medicare documentation system. Distinct sections and subsections are maintained for each of the four DMEPOS benefit categories. The coverage criteria for a benefit derive from the functional requirements or the definition of that benefit in the Social Security Act section 1861 or in the derivative definition in the MBPM.
| STATUTORYDMEPOS BENEFIT CATEGORIES |
|---|
| BENEFIT CATEGORY AUTHORITY |
SURGICAL DRESSINGS, SPLINTS, CASTS |
DURABLE MEDICAL EQUIPMENT |
PROSTHETIC DEVICES |
PROSTHETICS AND ORTHOTICS |
| Title XVIII Social Security Act (SSA) |
§1832(a)(1)
§1861(s)(5) |
§1832(a)(1)
§1861(s)(6)
§1861(n) |
§1832(a)(2)(I)
§1861(s)(8) |
§1832(a)(2)(I)
§1861(s)(9) |
| Code of Federal Regulations 42 CFR |
§410.36(a)(1) |
§410.38
§414.202 DME |
§410.36(a)(2)
§414.202 POD(1) |
§410.36(a)(3)
§414.202 POD(3) |
| Medicare Benefit Policy Manual Pub. 100-02, Ch. 15, Covered Services |
§100 |
§110
§110.1 |
§120 |
§130 |
| Medicare National Coverage Determinations Manual Pub. 100-03 |
§270 |
§280.1 |
§§20, 50, 80, 160, 180, 230 |
|
| Medicare Claims Processing Manual Pub. 100-04, Ch. 20 DMEPOS |
|
§10.1.1 |
§10.1.2 |
§10.1.3 |
| Example Local Coverage Determinations (LCDs) |
L33831 |
L33829
L33739 |
L33317
L33798 |
L33318
L33686
L33737
L33787 |
| HCPCS Code Group |
A-Code, S-Code |
E-Code |
L-Code, S-Code |
L-Code |
Table 1. Flow down of DMEPOS requirements
The NCDs and other program policies cited in Table 1 are all concerned with coverage within a benefit category, and trace their coverage criteria and instructions to the top-level benefit in the Social Security Act.
Title XVIII of the Act confers Medicare benefits. “Benefits provided to an individual” consist of “medical and other health services” which are defined in §1861 [reference §1832(a) and (b)] and which are not excluded by §1862, i.e. “items which are reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member.” [§1862(a)(1)(A)]
In addition to not being excluded by the “reasonable and medically necessary” criteria for coverage, the items must fall into a benefit category defined in §1861. Statutory references to the four benefit categories relevant to this issue are summarized in the Table above, together with the CMS Program Manuals and NCDs that allocate and expand the statutory requirements down to implementation instructions. These regulations and relevant NCDs are binding on all Medicare Contractors, QICs and ALJs. [42 C.F.R. §405.860(a)].
A coverage determination establishing coverage criteria for the items in one benefit category can exclude coverage of items within that category because they do not meet the criteria for that benefit category, but it cannot exclude coverage of an item covered in a different category since the coverage criteria are different for each category. Even within the same benefit category, there is a distinction between different functional utilization, e.g. the criteria for use of compression stockings for the treatment of venous ulcers are not applicable to the use of compression stockings for the treatment of burns.
Benefit Categories Are Defined by the Function, Not the Form, of the Items It is important to note that the definitions and coverage criteria contained in the Social Security Act (SSA) and in the Medicare Benefit Policy Manual (MBPM) are functional requirements. For example:
SSA §1861(s)(5) “…used for reduction of fractures and dislocations;”
SSA §1861(s)(8) “…which replace all or part of an internal organ…”
SSA §1861(n) “…used as a wheelchair…”, “…used in the patient’s home…”
MBPM §100 “…required for the treatment of a wound…”
MBPM §110 “…used to serve a medical purpose…”
MBPM §120 “…replace all or part of an internal body organ (including contiguous tissue), or replace all or part of the function of a permanently inoperative or malfunctioning internal body organ …”
MBPM § 130 “…used for the purpose of supporting a weak or deformed body member or restricting or eliminating motion in a diseased or injured part of the body…”
All definitions of items within one of the four DMEPOS benefit categories are defined by their medical function, and not their form.
CMS guidelines on the coverage of compression garments also recognize their medical function, to distinguish between benefit categories. Examples:
-
Program Memorandum Transmittal AB-03-090 June 30, 2003 and LCD for Surgical Dressings (L11471)- below the knee length compression stockings are covered when used as secondary surgical dressings in the treatment of venous stasis ulcers in accordance with SSA §1861(s)(5) —Coverage of compression garments in the treatment of venous Stasis Ulcers outlines coverage criteria for compression stockings when used in the treatment of venous stasis ulcers. Stockings used in this function were classified as prosthetic services, lower limb and originally given an HCPCS designation as L8110 and L8120.
-
-
LCD for Surgical Dressings (L11471) Compression burn garments are covered under the surgical dressings benefit when they are used to reduce hypertrophic scarring and joint contractures following a burn injury.? In this function they are given the HCPCS code group Compression Burn Garments (A6501-A6513).
The above two coverage descriptions are permissive in that they describe the functional uses of compression garments that meet coverage criteria under the surgical dressing benefit category. They are not prohibitive, i.e. they do not prohibit coverage when used in other medical functions and may fall into different benefit categories.
There is no LCD or NCD concerning coverage of compression garments used for treatment of lymphedema as prosthetic devices in accordance with SSA §1861(s)(8).
A specific item might meet the functional requirements of multiple benefit categories or none. Consider the functions of a compression garment:
-
Covered as a secondary wound dressing (Axxxx) [ICD-9-CM: 870-897]
-
Covered as a burn dressing (Axxxx) [ICD-9-CM: 940-949]
-
Covered as part of lymphedema pump (Exxxx) [ICD-9-CM: 457, 757]
When an item fails to meet the functional coverage requirements for a particular benefit category it can be excluded from that category because of its failure to meet the coverage criteria for that benefit category:
-
Non-covered as a leg brace (fabric supports not covered as an orthotic benefit)
-
Non-covered as durable medical equipment (not rentable)
-
Non-covered for leg fatigue (not medically necessary)
Non-coverage for one benefit category does not mean that its medical function does not qualify it for a different category.
Consider MLN Matters Number: MM6297 Related Change Request Number: 6297, 12/23/08
“Medicare Coverage of Elastic Support Garments
We have received questions regarding coverage of elastic support garments as leg, arm, back, or neck braces (orthotics). The definition of a brace in section 130 of Chapter 15 of the Medicare Benefit Policy Manual (Pub. 100-02) specifies that:
A brace includes rigid and semi-rigid devices which are used for the purpose of supporting a weak or deformed body member or restricting or eliminating motion in a diseased or injured part of the body. Elastic stockings, garter belts, and similar devices do not come within the scope of the definition of a brace.
Elastic garments or devices in general do not meet the definition of a brace because they are not rigid or semi-rigid devices. This includes devices that include stays that do not provide sufficient pressure to restrict or eliminate motion in the body part. While elastic devices may provide compression or warmth to a leg, arm, back, or neck, if they do not restrict or eliminate motion in a diseased or injured part of the body, then they may not be covered as braces.”
In section 280.1 of the Medicare National Coverage Determination Manual (Pub. 100-03) Elastic Stockings are listed as not covered as Durable Medical Equipment because they are not rental-type items per §1861(n) even though they meet all other definitions of DME in §1861(s)(6). But these same Elastic Support Garments are covered when used as secondary dressings or as burn coverings since they meet the coverage criteria for Surgical Dressings when used in a different medical function.
It is apparent in the above examples that CMS is generally very careful in considering the medical use of an item when applying coverage criteria for a specific benefit category, and the appropriate language is used in the applicable LCD.
Unintended Consequences of 2005 HCPCS Workgroup Recoding Action On May 24, 2005 the HCPCS Workgroup acted on a request to re-code Gradient Compression Stockings from their prosthetic services L-Codes to surgical dressing A-Codes [Appendix A]. The only rationale documented was that “These items are not orthotics, but some of the items are covered under the surgical dressing benefit.” This administrative change was made with no thought as to why these medical items were coded under lower limb prosthetic services (not orthotics, as stated) in the first place, and what the consequences of assigning them A-Codes might be. There was no public meeting and no opportunity for public or professional comment. Coverage for surgical dressing benefits requires that they be used in the treatment of open debrideable wounds. It is obvious that items in other benefit categories (e.g. DME, prosthetics, orthotics, prosthetic devices) are not required to meet the open wound coverage requirement. But from 12/31/05 to the present, compression stockings, whether their medical use met the coverage requirements of another benefit category or not, were given an A-Code and rejected by authority of the LCD for Surgical Dressings. This administrative change has been responsible for the denial of appropriate medically necessary treatment for hundreds, perhaps thousands, of Medicare beneficiaries with lymphedema.
In late 2006 Mr. Robert Weiss submitted a formal request to the HCPCS Workgroup to restore the L-Codes for compression garments used in the treatment of lymphedema since lymphedema compression garments were beginning to be denied on the basis that they did not meet the coverage requirements of surgical dressings as stated in the LCD for Surgical Dressings. The HCPCS Workgroup denied the request on the basis that “No insurer (i.e., Medicare, Medicaid, Private Insurance Sector) identified a national program operating need to establish unique codes to distinguish all the products listed in this application. Existing codes adequately describe the array of products available.” It has been obvious to Medicare beneficiaries with lymphedema that there are not adequate codes to describe their medically required compression items, and that the miscoding of what items that may be described precludes them from being covered. It has been obvious to the insurance sector as well, and they have had a separate S-Code group created in 2005 for upper limb compression garments as well as short-stretch bandages and padding for lymphedema since no properly-coded and described items existed for their operating need.
Medicare contractors, healthcare providers, as well as insurance contractors have expressed the need to change the HCPCS codes associated with compression therapy for lymphedema. Milliman Care, CIGNA Healthcare, Excellus BCBS, Kaiser Permanente and others have issued coverage guidelines that list compression garments used in the treatment of lymphedema as “prosthetic devices” or “prosthetics and orthotics”, in contradistinction to the use of similar compression garments in the treatment of wounds.
No codes exist for some of the items commonly used in lymphedema treatment, such as compression vests and bras, compression calibration gages, directional flow pads and garments, external compression devices, compression bandaging kits, etc.
Definition of “Prosthetic Device” Benefit Category [CMS 100-02] Prosthetic devices are items which replace all or part of an internal body organ or replace all or part of the function of a permanently inoperative or malfunctioning internal body organ. The test of permanence is considered met if the medical record, including the judgment of the attending physician, indicates that the condition is of long and indefinite duration.
In addition to artificial arms and legs, coverage under this benefit includes, but is not limited to, breast prostheses, eye prostheses, parenteral and enteral nutrition, ostomy supplies, urological supplies in patients with permanent urinary incontinence, and glasses or contact lenses in patients with aphakia or pseudophakia.
Supplies that are necessary for the effective use of a medically necessary prosthetic device are covered. Equipment, accessories and supplies (including nutrients), which are used directly with an enteral or parenteral nutrition device to achieve the therapeutic benefit of the prosthesis or to assure the proper functioning of the device, are covered.
Repairs, adjustments and replacement of medically necessary prosthetic devices are covered. Dental prostheses (i.e., dentures) are excluded from coverage. Claims for internal prostheses (e.g., intraocular lens, joint implants, etc.) are not processed by the DME MAC.
Parsing the Coverage Requirements for Prosthetic Devices “Prosthetic devices are items which … replace all or part of the function of a permanently inoperative or malfunctioning internal body organ.”
Internal Body Organ. An organ system is a group of anatomical structures that work together to perform a specific function or task. Although we learn about each organ system as a distinct entity, the functions of the body's organ systems overlap considerably, and your body could not function without the cooperation of all of its organ systems. In fact, the failure of even one organ system could lead to severe disability or even death.
The human body is composed of 11 different organ systems. These include the following:
-
Integumentary
-
Muscular
-
Skeletal
-
Nervous
-
Circulatory
-
Lymphatic
-
Respiratory
-
Endocrine
-
Urinary/excretory
-
Reproductive
-
Digestive
Some scientists add the immune system to this list to make a total of 12 organ systems, but most people consider the immune system to be a part of the lymphatic system. You may also find texts where the lymphatic and immune systems are both included within the circulatory system, which would give us a total of ten organ systems. Still other sources separate the immune system, the vestibular system (the organs of balance) and the neurotransmitter system (chemicals that control our moods, memory, appetite, sleep, etc.) from the other organ systems, which would spawn 13 organ systems. [Study.com 2016]
Definition and Function of the Lymphatic System. The lymphatic system is a system of capillaries, vessels, nodes and other organs that transport a fluid called lymph from the tissue interstitium and returns it into the bloodstream. The lymphatic tissue of these organs filters and cleans the lymph of any debris, abnormal cells, or pathogens. The part of this body system that is relevant to this discussion is the network of lymphatic vessels that collect interstitial fluids (lymph) and transport these fluids to the heart.
Pathophysiology of lymphedema. Lymphedema, also called “lymph stasis”, can be defined as the tissue fluid accumulation that arises as a consequence of impaired lymphatic drainage. The reduced drainage of fluid from the tissues can be the result of inability of collecting lymphatics to collect tissue fluid, interruption or blockage of the drainage network, impaired functionality of the lymphatic network to transport lymph to functional parts of the system, or venous hypertension opposing the delivery of lymph to the venous system.
Lymphedema is a Permanent Malfunctioning of Part of the Lymphatic System. When lymphedema is not treated, the protein-rich fluid continues to accumulate, leading to even more swelling and hardening of the tissue, referred to as fibrosis. This stagnant fluid is a good culture medium for bacteria, often resulting in recurring infections when there are injuries to the skin, decrease or loss of functioning of the affected limbs, and skin breakdown. Infections to the lymphatic system (lymphangitis) and to the skin (cellulitis) can affect the connective tissue of the skin. Repeated infections may result in scarring, making the skin susceptible to even more swelling and infections. Over time the skin tissue becomes hard (fibrotic)—a characteristic of severe chronic lymphedema. In very severe cases, untreated lymphedema may even result in a rare form of lymphatic cancer called lymphangiosarcoma.
Role of Compression in Replacing the Function of a Locally Malfunctioning Lymphatic System. Rationale for compression therapy [Cavezzi 2004]: Compression therapy (bandage, stockings and sleeves) have some documented positive actions on lymphoedema: a) reduction of limb volume, through several mechanisms: increase of interstitial (transmural) pressure, increase of protein and fluid recovery in the lymphatic network, increase of lymphangion contractility, shift of fluids from affected to non- (or hypo-) affected proximal areas of the limb, b) improvement of musculo-vascular foot/calf pump, c) protection of the skin (easily prone to infections etc.) Multi-layer short-stretch bandaging is the favourite treatment over elastic stockings/sleeves in the intensive care phase. Compression/sleeves are mainly used for the maintenance phase. The scientific literature is replete with references on the physiological mechanisms by which compression helps the deficient lymphatic system to regain its function of collecting and transporting lymph fluid. In a gross sense, the lymphatic system of a lymphedematous extremity is incapable of collecting and transporting the excess tissue fluid, leading to swelling and destructive, progressive tissue changes and frequent infections. With the daily use of compression, swelling is controlled, tissue changes are retarded or eliminated, and frequency of infections reduced significantly. The beneficial result of compression was verified by the MEDCAC Lymphedema Expert Panel [MEDCAC 2009]. This data alone should be adequate to justify coverage of compression items for the treatment of lymphedema. But a more detailed look must be taken to show how compression enables the functioning of the permanently malfunctioning lymphatic system, and how it meets the coverage criteria for prosthetic devices.
The role of compression in the restoration of lymphatic flow in a malfunctioning part of the lymphatic system may be understood by inspecting the mechanisms of compression and the effect of each mechanism. Table 2 is a summary chart taken from Partsch 2006 explaining the mechanisms by which compression serves to restore the faulty local collection and transport functions of the permanently malfunctioning lymphatics in lymphedema.
| Mechanism | Effect |
|---|
| Increased interstitial pressure |
Reduced capillary filtration and production of lymph; limb volume decrease [Bates 1992, Partsch 2003, Levick 2003] |
| Shift of fluid into uncompressed areas |
Proximal volume increase accommodated by normally working lymphatics in that region and assisted by manual lymphatic drainage [Földi 1983] |
| Increased lymph reabsorption and stimulation of lymphatic contractions |
Improvement of lymph kinetics as shown by lymphoscintigraphy and intralymphatic measurement of flow and pressure [Leduc 1988] |
| Breakdown of fibrosclerotic tissue |
Softening of tissue as shown by ultrasound [Gniadecka 1995] and durometer [Falanga 1993] |
| Improvement of venous pump in patients with venolymphatic dysfunction |
Increased expelled blood volume; reduction of venous reflux and ambulatory venous hypertension [Partsch 1984] |
Table 2 Mechanisms and effects of compression therapy in lymphoedema, Partsch 2006
Reduction in capillary filtration. Lymph originates as a clear, protein-rich fluid in tissue spaces throughout the body. This fluid (1 -2 liters/day) is usually carried by lymph vessels, passes through regional lymph nodes and joins the venous blood shortly before this blood enters the heart. The circulation of lymph is important in maintaining normal tissue homeostasis throughout the body [Klose 1998].
The balance between the fluid leaving the arterial side of the capillaries (ultrafiltration) on the one hand and the reabsorbtion that occurs in the venous capillaries plus the drainage via the lymphatics on the other, is known as “Starling's equilibrium". Because of the disturbed lymph drainage in lymphedema, this equilibrium collapses, protein-rich fluid accumulates in the tissue spaces, the colloid-osmotic pressure rises and all of these events favor ultrafiltration. Raising the tissue (interstitial) pressure by means of an external force (the compression bandage), reduces the effective ultrafiltration pressure, less fluid accumulates and less fluid has to be removed from the tissue spaces. The lymphedema improves [Klose 1998].
Starling’s hypothesis states that the exchange of water and small molecules is governed mainly by the transcapillary hydrostatic and colloid osmotic pressures. External compression increases the interstitial pressure and prevents fluid from filtering out of the capillary network [Bates 1992, Partsch 2003, Levick 2003]. The role of capillary reabsorption is still a matter of debate [Levick 2003]. Compression removes more water than protein from the tissue, increasing oncotic tissue pressure and reinforcing the need for sustained compression [Partsch 1981] In chronic lymphoedema, therefore, success depends on continued compression.
Shift of fluid into non-compressed parts of the body. Oedematous fluid may be shifted into non-compressed distal and proximal parts of the body. Bandaging the fingers and toes and, as far as possible, using compression devices to cover proximal, non-oedematous areas of an extremity should prevent this. Manual lymphatic drainage is especially important in patients with proximal lymphoedema.
A higher intravasal pressure in the lymphangion improves the function of existing axillo-axillary, axillo-inguinal and inguino-inguinal anastomoses. In addition, accessory lymph collectors develop and compensatory dilatation of cutaneous lymphatics crossing the lymphatic watershed may be observed [Kubik 1986 and Kubik 1999].
Increase in lymphatic reabsorption and stimulation of lymphatic transport. Decongestive lymphatic therapy reduces microlymphatic hypertension and improves microlymphatic dynamics in patients with lymphoedema of the lower limbs. It has been shown that microlymphatic pressure can be nearly normalised with at least two weeks of intensive manual lymphatic drainage and multi-layer short-stretch bandaging [Franzeck 1997]. In this study a reduction in the maximum dispersion of a fluorescent dye indicated that drainage from the superficial lymph capillaries had improved.
Olszewski cannulated superficial lymph collectors in lymphoedematous and healthy human legs [Olszewski 1991]. Using a pressure recorder, flow meter and test tube to collect lymph, the influence of an elastic bandage and contracting calf muscle (exercise) on lymph pressure and flow were measured. The results showed that both compression bandaging and exercise stimulated the movement of stagnating lymph through the lymph collector in lymphoedema patients, in which the lymphatic trunks are filled, but not in healthy individuals, in which they are empty. This supports the conclusion that compression is likely to be more effective when the intrinsic pump fails than when it is functioning normally [Levick 2003].
Lymphoscintigraphy has been used to demonstrate an immediate increase in lymph drainage after manual lymphatic drainage [Leduc 1988] and the initiation of intermittent pneumatic compression devices [Partsch 1981, Leduc 1988]. If compression bandaging is continued (using interface pressure 40–60mmHg), the dynamics of the cutaneous lymphatic circulation remain improved [Casley-Smith 2000]. However, lymphoscintigraphy has shown that several weeks of compression therapy improve disturbed lymph drainage only in some cases and not in patients with severe, indurated lymphoedema [Földi 1985].
Improvement in the venous pump in patients with veno-lymphatic dysfunction. In cases with accompanying venous insufficiency compression therapy reduces venous reflux and promotes venous return. Inelastic, short-stretch bandages applied with tension to deliver a high pressure can reduce ambulatory venous hypertension [Partsch 1984], resulting in a reduction in capillary hypertension [Jünger 2000] and consequent reduction in the lymphatic load. Subfascial lymph transport is decreased in patients with deep vein thrombosis and post thrombotic syndrome [Lofferer 1972], but this can be improved by the application of inelastic bandages [Haid 1968].
Bandaging improves the Efficiency of the Muscle and Joint Pumps. Lymph is propelled through the various lymph vessels by muscular activity, by contraction of the lymph vessels themselves, by the movements of the diaphragm (breathing) and by negative pressures within the chest during the breathing cycle. In the extremities the activity of the skeletal muscles is an important factor in lymph transport. During contraction of an arm or a leg muscle, the venous and lymphatic systems propel the fluids they contain towards the heart. This results in a more rapid flow and a decompression of both systems. In order to preserve this process, normal tissue (skin) and joints are essential.
In lymphedema these conditions are damaged. The skin is more or less overstretched and, especially after any decongestion of the lymphedema has been achieved, skin and tissue pressures are even further diminished. The use of external compression bandages compensates for this diminished tissue and skin pressure and thus improves the efficiency of the muscle and joint pumps [Klose 1998]. The skin and bandage offer firm resistance to the contracting muscles, so that muscle and joint pumps become more effective. Only heavy-duty bandages are capable of providing the muscles with the appropriate support [Schingale 1996].
The compression bandage reduces the lumen of the lymphatic vessels and accelerates the flow rate. Where there is valvular insufficiency - including defective venous valves - the defective valves are partly closed by reducing the lumen and adequate valve closure is achieved [Schingale 1996].
Breakdown of fibrosclerotic tissue. Although the mode of action at the molecular level is not clear, compression also helps to break down fibrosclerosis. In patients with venous leg ulceration compression accelerates blood flow in the microcirculation, favours white cell detachment from the endothelium and prevents further adhesion of the neutrophils. In lipodermosclerotic areas where skin perfusion may be reduced due to the strain associated with high tissue pressure, compression therapy can increase this gradient and improve blood flow. This leads to softened skin.
In a clinical trial the quantitative expression of the genes encoding CD14, interferon-γ receptor (IFNγR), tumour necrosis factor-alpha (TNF-α), very late antigen-4 (VLA-4), TNF receptor-1 (TNFR1) and CD44 was examined in patients with peripheral leg lymphoedema to investigate the potential role of gene expression in the inflammatory response [Földi 2000]. The findings suggest that proinflammatory cytokines and receptors for growth factors are upregulated in patients with primary and secondary lymphoedema and become downregulated after decongestive lymphatic therapy using a combination of skin care, manual lymphatic drainage, compression therapy and remedial exercises. It seems reasonable to assume that the development of fibrosclerosis in these patients is triggered by the dysregulation of these molecular mechanisms.
Experimental investigations have demonstrated that lymph drainage is accompanied by increased macrophage activity that should result in increased interstitial protein degradation and, therefore, reduced volume of lymph transported material. Investigations concerning the effectiveness of drug treatment i.e. with benzopyrones, to increase macrophage activity are contradictory [Weissleder 2001]
Compression therapy, initially by individual bandaging and then by customized compression stockings, increases interstitial tissue pressure. The resulting better filling of the initial lymphatics causes edema reduction. At the same time, atrophy of the collagenous connective tissue results in a corresponding volume reduction [Weissleder 2001].
Coverage Logic is Parallel to Coverage of Scleral Shells as Prosthetic Devices A similar logic is used in the coverage of a scleral shell or scleral shield. Scleral shells are covered as prosthetic devices when they are used in the medical treatment of “dry eye”. [NCD 80.5]
Scleral shells are occasionally used in combination with artificial tears in the treatment of "dry eye" of diverse etiology. Tears ordinarily dry at a rapid rate, and are continually replaced by the lacrimal gland. When the lacrimal gland fails, the half-life of artificial tears may be greatly prolonged by the use of the scleral contact lens as a protective barrier against the drying action of the atmosphere. Thus, the difficult and sometimes hazardous process of frequent installation of artificial tears may be avoided. The lens acts in this instance to substitute, in part, for the functioning of the diseased lacrimal gland and would be covered as a prosthetic device in the rare case when it is used in the treatment of "dry eye."
The Medicare Benefit Policy Manual, Chapter 15, “Covered Medical and Other Health Services,” §§120 and 130.
In this case they are coded as L9900 Orthotic and prosthetic supply, accessory, and/or service component of another HCPCS “L” code. They do not replace the diseased lacrimal gland, but acts as a substitute, in part, for the function of the gland.
In a like manner compression bandages and garments substitute, in part, for the functions of the lymphatic system in enabling collection of tissue fluid from the interstitial space and transporting it to functioning nodes to be filtered and returned to the circulatory system. The compression items do not replace missing or non-functioning lymph nodes, but they enable the affected lymphatics to resume their intended functions of collecting and transporting tissue fluids.
Prosthetic devices that replace an internal body organ or replace the function of an internal body organ may be internal or external. If internal, they are covered by Medicare and bundled with the surgery that implants them. If external they are covered by Medicare as separate DMEPOS benefits.
8. CLARIFICATIONS/CHANGES TO EXISTING MEDICARE DOCUMENTS
The proposal for coverage of compression items used in the compression therapy for lymphedema as prosthetic devices does not require a change to any Medicare law or statute. It requires a new look at these enabling and governing statutes from a legal and from a medical logic to determine whether this vital coverage may be within both the letter and the spirit of title XVIII of the Act. Dozens of Administrative Law Judges have already reviewed the facts and have agreed the medical use of these compression items in the treatment of lymphedema warrants their coverage as prosthetic devices under current Medicare law. It remains for CMS to determine whether or not an unbiased interpretation of the evidence supports or refutes the requested coverage in light of the great advances in our knowledge of the lymphatic system and in the treatment technology gained in the last decade.
Changes might have to be made to NCDs §280.1, 280.6 and 270.5 to better explain the concept of benefit categories, and that, depending on the medical usage of an item, it might be covered as a surgical dressing, a burn garment or as a lymphedema compression garment so long as it meets the coverage criteria for one of the DMEPOS benefit categories. Similar changes to the LCD for Surgical Dressings would be made explaining that although lymphedema garments and bandages may not be covered as surgical dressings, they may meet the coverage criteria for prosthetic devices and supplies and be covered by Medicare.
Since this constitutes a new interpretation of Medicare statutes, it might be appropriate to elaborate on the medical necessity explanations and billing changes for lymphedema compression therapy items in a new prosthetic devices LCD and LCA covering compression items used in lymphedema compression therapy.
And lastly, CMS should reconsider creating new HCPCS L-Codes for these lymphedema compression items recognizing that their medical use qualifies them for coverage as prosthetic devices under §1861(s)(8) as interpreted by the Medicare Benefit Policy Manual, Chapter 15, §120 Prosthetic Devices. As a minimum the compression stockings which were removed as L-Codes L8100-L8239 and made A-Codes A6530-A6549 in 2006 should be restored, and the S-Codes S8420-S8431 used by the insurance industry for lymphedema sleeves and lymphedema bandages and padding should be given L-Codes in recognition of their new coverage status as prosthetic devices. CMS may wish to reconsider the strawman extended L-Code list first presented to the HCPCS Work Group in 2007 and summarized as Appendix C.
9. REFERENCES
ACS 1998: American Cancer Society “Workshop III Diagnosis and Management of Lymphedema”, Cancer Supplement December 15, 1998; 83(12):2882-5.
Aetna 2014: Aetna Clinical Policy Bulletin: “Compression Garments for the Legs”, CPB 0482, Aetna, Inc., 4/5/2014.
Aetna 2015: Aetna Clinical Policy Bulletin: “Lymphedema”, CPB 0069, Aetna, Inc., 01/09/2015.
Balzer 1993: Balzer K & Schonebeck I: [Edema after vascular surgery interventions and its therapy] in German Z Lymphol. 1993 Dec;17(2):41-7.
Bar Ad 2009: Bar Ad V, Cheville A, Solin LJ et al: "Time course of mild lymphedema after breast conservation treatment for early-stage breast cancer" Int J Radiation Biol Phys. 2009;76(1):85-90.
Bates 1992: Bates DO, Levick JR, Mortimer PS. “Subcutaneous interstitial fluid pressure and arm volume in lymphoedema” Int J Microcirc Clin Exp 1992;11(4):359-73.
Boris 1997: Boris M, Weindorf S & Lasinski B: “Persistence of Lymphedema Reduction After Complex Lymphedema Therapy”, Oncology (Huntingt) Jan 1997;11(1):99-109, discussion 110,113-4.
Boris 1998: Boris M, Weindorf S & Lasinski B: "The risk of genital edema after external pump compression for lower limb lymphedema " Lymphology Mar 1998; 31(1):15-20.
Bosompra 2002: Bosompra K, Ashikaga T, O'Brien PJ, Nelson L, Skelly J, Beatty, DJ: "Knowledge about preventing and managing lymphedema: a survey of recently diagnosed and treated breast cancer patients", Patient Edu Couns. June 2002:47(2);155-63.
Box 2002: Box RC, Reul-Hirche HM, Bullock-Saxton JE, et al.: "Physiotherapy after breast cancer surgery: Results of a randomized controlled study to minimize lymphoedema" Breast Cancer Res Treat. 2002;75:51-64.
Brayton 2014: Brayton KM, Hirsch AT, O’Brien PJ, Cheville A, Karaca-Mandic P and Rockson SG. “Lymphedema prevalence and treatment benefits in cancer: Impact of a therapeutic intervention on health outcomes and costs” PLoS ONE 9(12): e114597.
Britannica 2015: The Editors of Encyclopedia Britannica. “Lymphatic System, Anatomy” http://www.britannica.com/science/lymphatic-system [Accessed December 22, 2015].
Burdette 2005: Burdette SD and Bernstein JM. “The Gift That Keeps on Giving” SKINmed: Dermatology for the Clinician Nov-Dec 2005:381-4.
Campisi 2003: Campisi C, Boccardo F, Zilli A, et al. “Peripheral lymphedema: new advances in microsurgical treatment and long-term outcome.” Microsurgery 2003;23:522.
Casley-Smith 2000: Casley-Smith JR. “Changes in the microcirculation at the superficial and deeper levels in lymphoedema: the effects and results of massage, compression, exercise and benzopyrones on these levels during treatment”. Clin Hemorheol Microcirc 2000; 23(2-4): 335–43.
Cavezzi 2004: Cavezzi A. “Lymphoedema Section 3.1” in Partsch H. Editor, “Evidence based compression therapy” in VASA J. Vascular Diseases December 2003;32 Supp 63:17.
Chikly 2001: Chikly B: “External Medical Compression” Part Four, Chapter 6 in Silent Waves. Theory and practice of Lymph Drainage Therapy I.H.H. Publishing, Scottsdale, Arizona, 2001:261-275.
Clarke 1982: Clarke D, Martinez A, Cox RS & Goffinet DR: “Breast edema following staging axillary node dissection in patient with breast carcinoma treated by radical radiotherapy” Cancer Jun 1, 1982;49(11): 2295-9.
CMS 100-02: Medicare Benefits Policy Manual, Chapter 15, §120 Prosthetic Devices.
CMS 100-16: Medicare Managed Care Manual, Chapter 17f, §10.1
CMS 2013: Jimmo v. Sebelius Settlement Agreement Fact Sheet
Coblentz 2002: Coblentz TR & Theodorescu D: “Morbidity of modified prophylactic inguinal lymphadenectomy for squamous cell carcinoma of the penis” J Urol. Oct 2002;168(4 Pt 1):1386-9.
Damstra 2008: Damstra RJ, van Steensel MA, Boomsma JH, Nelemans P & Veraart JC: "Erysipelas as a sign of sub-clinical primary lymphoedema: a prospective quantitative scintigraphic study of 40 patients with unilateral erysipelas of the leg" Br J Dermatol. Jun 2008;158(6):1210-5.
DME MAC A Supplier Manual: Chapter 12, Medical Review, Local Coverage Determinations.
Falanga 1993: Falanga V, Bucalo B. “Use of a durometer to assess skin hardness” J Am Acad Dermatol 1993; 29(1): 47-51.
Fehlauer 2003: Fehlauer F, Tribius S, Höller U, Rades D, Kuhlmey A, Bajrovic A and Alberti W: "Long-term radiation sequelae after breast-conserving therapy in women with early-stage breast cancer: An observational study using the LENT-SOMA scoring system." Int J Radiation Oncology Biol Phys, 2003;55(3):651-8.
Földi 1983: Földi E, Földi M. “Die Anatomischen Grundlagen der Lymphödembehandlug” Schweiz Runsch Med Prax. 1983;72(46):1459-64.
Földi 1985: Földi E, Földi M, Weissleder H. “Conservative treatment of lymphoedema of the limbs”. Angiology 1985; 36(3): 171–80.
Földi 1996: Földi E: "Prevention of Dermatolymphangioadenities by combined physiotherapy of the Swollen Arm after Treatment of Breast Cancer", Lymphology, 1996;29:48-49.
Földi 1998: Földi E. "The treatment of lymphedema" Cancer 1998;15(12 Supplement):2833-34.
Földi 2000: Földi E, Sauerwald A, Hennig B. “Effect of complex decongestive physiotherapy on gene expression for the inflammatory response in peripheral lymphedema”. Lymphology 2000; 33(1): 19–23.
Földi 2005: Földi E, Jünger M and Partsch H. “The science of lymphoedema bandaging” in EuropeanWound Management Association (EWMA). Focus Document: Lymphoedema bandaging in practice. London: MEP Ltd, 2005.
Franzeck 1997: Franzeck UK, Spiegel I, Fischer M, et al. “Combined physical therapy for lymphoedema evaluated by fluorescence microlymphography and lymph capillary pressure measurements” J Vasc Res1997;34(4):306-11.
Gaarenstroom 2003: Gaarenstroom KN, Kenter GG, Trimbos JB, Agous I, Amant F, Peters AA & Vergote I: “Postoperative complications after vulvectomy and inguinofemoral lymphadenectomy using separate groin incisions” Int J Gynecol Cancer. Jul-Aug 2003;13(4):522-7.
Gniadecka 1995: Gniadecka M. “Dermal oedema in lipodermatosclerosis: distribution, effects of posture and compressive therapy evaluated by high-frequency ultrasonography” Acta Derm Venereol 1995;75(2): 120-24.
Goffman 2004: Goffman TE, Laronga C, Wilson L & Elkins D: “Lymphedema of the arm and breast in irradiated breast cancer patients: risks in an era of dramatically changing axillary surgery” Breast J. Sep –Oct 2004;10(5):405-11.
Haid 1968: Haid H, Lofferer O, Mostbeck A, Partsch H. [Lymph kinetics in the postthrombotic syndrome under compression bandages]. Med Klin 1968; 63(19): 754-57.
HCPCS 2003: Department of Human Services HCPCS Manual July 2003.
Højris 2000: Højris I, Anderson J, Overgaard M & Overgaard J.: "Late treatment-related morbidity in breast cancer patients randomized to postmastectomy radiotherapy and systemic treatment versus systemic treatment alone", Acta Oncol. 2000;39(3):355–72.
Hutchison 2010: Hutchison NA, Letter to Congressman Larry Kissell, August 21, 2010.
ISL 2013: Executive Committee, International Society of Lymphology, “The diagnosis and treatment of peripheral lymphedema: 2013 consensus document of the International Society of Lymphology” Lymphology 2013;46:1-11.
James 1982: James JH: “Lymphoedema following ilio-inguinal lymph node dissection” Scand J Plast Reconstr Surg. 1982;16(2):167-71.
Jünger 2000: Jünger, Steins A, Hahn M, Hafner HM. “Microcirculatory dysfunction in chronic venous insufficiency (CVI)”. Microcirculation 2000; 7(6 Pt 2): S3-12.
Kasseroller 1998: Kasseroller RG: "Sodium selenite as prophylaxis against erysipelas in secondary lymphedema", Anticancer Res May-Jun1998;18(3C)2227-30.
Kissin 1982: Kissin MW, Thompson EM, Price AB & Slavin G: “The inadequacy of axillary sampling in breast cancer” Lancet May 29, 1982;1(8283):1210-2.
Klose 1998: Klose G: Lymphedema Bandaging. Practical Bandaging Instructions for Lymphedema Patients and Therapists Lohmann & Rauscher GmbH, Neuwied, Ger. 1998:11.
Ko 1998: Ko DSC, Lerner R, Klose G & Cosimi AB: “Effective Treatment of Lymphedema of the Extremities”, Arch Surg. Apr 1998;133(4):452-8.
Kubik 1986: Kubik S, Manestar M. “Anatomische Grundlagen der Therapie des Lymphödems”. Ödem 1986:19-31.
Kubik 1999: Kubik S. “Anatomie des Lymphgefässystems” In: Fö1di M, Kubik S. eds. Lehrbuch der Lymphologie (3rd ed.). Stuttgart-New York: G. Fischer 1999;1-201.
Laidley 2016: Laidley A and Anglin B. “The impact of L-Dex® measurements in assessing breast cancer-related lymphedema as part of routine clinical practice”. Front Oncol. Sept 5, 2016. doi: 10.3389/fonc.2016.00192. [Epub ahead of print]
Lawenda 2010: Lawenda BD, Mondry TE and Peter & Johnstone PAS. “Lymphedema: A Primer on the Identification and Management of a Chronic Condition in Oncologic Treatment” CA Cancer J Clin. 2009;59:8-24.
Lawrance 2008: Lawrance S. “Use of a Velcro® Wrap system in the management of lower limb lymphoedema/chronic oedema” J Lymphoedema 2008;3(2):65-70.
Leduc 1988: Leduc O, Bourgeois P, Leduc A. “Manual lymphatic drainage: Scintigraphic demonstration of its efficacy on colloidal protein resorption” In: Partsch H (Ed). Progress in Lymphology XI. Amsterdam: Excerpta Medica, 1988;551-4.
Leduc 1988: Leduc A, Bastin R, Bourgeois P. “Lymphatic reabsorption of proteins and pressotherapies”. In: Partsch H (ed). Progress in Lymphology XI. Amsterdam: Excerpta Medica, 1988; 591-92.
Lee 2014: Lee BB, Antignani PL, Baroncelli TA, Boccardo FM, Brorson H, Campisi C, Damstra RJ, Flour M, Giannoukas A, Laredo J, Liu NF, Michelini S, Piller N, Rockson SG, Scuderi A, Szolnoky G, Yamamoto, T. “IUA-ISVI consensus for diagnosis guideline of chronic lymphedema of the limbs” Int Angiol. March 18, 2014.
Levick 2003: Levick JR. An Introduction to Cardiovascular Physiology London: Arnold, 2003.
Levick 2003: Levick JR, McHale N. “The physiology of lymph production and propulsion”. In: Browse N, Burnand K, Mortimer P (eds). Diseases of the Lymphatics. London: Hodder Arnold, 2003; 44-64.
Lieskovsky 1980: Lieskovsky G, Skinner DG & Weisenburger T: “Pelvic lymphadenectomy in the management of carcinoma of the prostate.” J Urol Nov 1980;124(5):635-8.
Lofferer 1972: Lofferer O, Mostbeck A, Partsch H. [Nuclear medicine diagnosis of lymphatic transport disorders of the lower extremities]. Vasa 1972; 1(2): 94-102.
Mallon 1997: Mallon E, Powell S, Mortimer P, & Ryan TJ: "Evidence for altered cell-mediated immunity in postmastectomy lymphoedema" Br J Dermatol Dec1997;137(6):928-33.
Mayo 2015: Mayo Clinic definition of Lymphedema at http://www.mayoclinic.org/diseases-conditions/lymphedema/basics/definition/con-20025603 (Accessed February 24, 2015)
MEDCAC 2009: Posted Score sheet from Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) meeting on lymphedema, November 18, 2009, Baltimore, MD http://www.cms.gov/Regulations-and-Guidance/Guidance/FACA/downloads/id51a.pdf
Micucci 2015: Micucci S. “Lymphedema Management and the PT’s Role: Lymphedema is a progressive disorder, and comprehensive management requires an interdisciplinary effort.” Advance Healthcare Network for Physical Therapy & Rehab Medicine, Posted on April 27, 2010 on http://physical-therapy.advanceweb.com/Features/Article-1/Lymphedema-Management-and-the-PTs-Role.aspx [Accessed December 13, 2015]
Mortimer 2014: Mortimer PS and Rockson SG. “New developments in clinical aspects of lymphatic disease” J Clin Inv. March 2014;124(3):915-21. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3938261/
Nelson 2004: Nelson BA, Cookson MS, Smith JA & Chang SS: “Complications of inguinal and pelvic lymphadenectomy for small squamous cell carcinoma of the penis: A contemporary series” J Urol. Aug 2004;172(2):494-7.
NLN 2011: NLN Advisory Committee. “Position Statement of the National Lymphedema Network, Topic: The Diagnosis and treatment of Lymphedema”, February 2011. http://lymphnet.org/pdfDocs/nlntreatment.pdf [Accessed January 18, 2015}
Ochalek 2014: Ochalek K, Gradalski T, and Szygula Z. “Five-Year Assessment of Maintenance Combined Physical Therapy in Postmastectomy Lymphedema” Lymphatic Research and Biology. Not available-, ahead of print. doi:10.1089/lrb.2014.0027. Online Ahead of Print: December 19, 2014.
Olszewski 1991: Olszewski WL. “Lymph pressure and flow in limbs” In: Olszewski WL (Ed). Lymph Stasis: pathophysiology, diagnosis and treatment. Boca Raton, FL: CRC Press, 1991:109-55.
Papachristou 1977: Papachristou D & Fortner JG: “Comparison of lymphedema following incontinuity and discontinuity groin dissection” Ann Surg. Jan 1977;185(1):13-6.
Partsch 1981: Partsch H, Mostbeck A, Leitner G. [Experimental studies on the efficacy of pressure wave massage (Lymphapress) in lymphedema]. Z Lymphol 1981; 5(1): 35-39.
Partsch 1984: Partsch H. [Improving the venous pumping function in chronic venous insufficiency by compression as dependent on pressure and material]. Vasa 1984;13(1):58-64.
Partsch 2003: Partsch H. “Understanding the pathophysiological effects of compression” In: European Wound Management Association (EWMA). Position Document: Understanding compression therapy. London: MEP Ltd, 2003:2-4.
Partsch 2006: Partsch H & Jünger M: “Evidence for the use of compression Hosiery in lymphoedema” in Lymphoedema Framework: Template for Practice: Compression Hosiery in Lymphoedema London MEP Ltd. 2006. Table 1 Mechanisms and effects of compression therapy in lymphoedema.
Peninsula 2015: “Tissue Gradient Technology—The ReidSleeve® Classic” Peninsula Medical, Inc. http://www.reidsleeve.com/rsleeve.htm [accessed January 17, 2015]
Ridner 2013: Ridner SH. “Pathophysiology of Lymphedema”, Seminars in Oncology Nursing February 2013;29(1):4-11.
Rogan 2016: Rogan S, Taeymans J, Luginbuehl H, Aebi M, Mahnig S & Gebruers N. “Therapy modalities to reduce lymphoedema in female breast cancer patients: a systematic review and meta-analysis” Breast Cancer Res Treat. Published On-Line 26 July 2016, DOI 10.1007/s10549-016-3919-4.
Rönkä 2004: Rönkä RH, Pamilo MS, von Smitten KAJ & Leidenius MHK: “Breast lymphedema after breast conserving treatment” Acta Oncol. Sep 2004;43(6):551-7.
Schingale 1996: Schingale F-J : “Compression Therapy” in Daróczy J, Schingale F-J & Mortimer PS: Practical Ambulant Lymphology Munich, Verlag medical concept GmbH, 1996:26-30.
Schünemann 1997: Schünemann H & Willich N: "Lymphödeme nach Mammakarzinom–Eine Studie über 5868 fälle" [Lymphedema after breast carcinoma. A study of 5868 cases] in German Dtsch Med Wochenschr. Apr 25,1997;122(17):536-41.
Senofsky 1991: Senofsky GM, Moffat FL, Davis K, Masri MM, Clark KC, Robinson DS, Sabates B & Ketcham AS. “Total axillary lymphadenectomy in the management of breast cancer” Arch Surg Nov 1991;126(11):1336-41, discussion 1341-2.
Shih 2009: Shih Y-CT, Xu Y, Cormier JN, et al: "Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study" J Clin Oncol. April 20, 2009;27(12):2007-14.
Smeltzer 1985: Smeltzer DM, Stickler GB & Schirger A: “Primary lymphedema in children and adolescents: a follow-up study and review” Pediatrics. Aug 1985;76(2):206-18.
Stoberl 1987: Stoberl C & Partsch H: “Erysipel und Lymphodém: Ei oder henne?” [Erysipelas and lymphedema: egg or hen?] in German Z Hautkr. 1987;62:56–62.
Stout 2008: Stout-Gergich NL, Pfalzer LA, McGarvey C et al: "Preoperative assessment enables the early diagnosis and successful treatment of lymphedema" Cancer 2008;112:2809-19.
Stout 2012: Stout NL, Pfalzer LA, Springer B, Levy E, McGarvey CL, Danoff JV, Gerber LH and Sobelle PW. “Breast cancer-related lymphedema: Comparing direct costs of a prospective surveillance model and a traditional model of care” Phys Ther. January 2012;92(1):152-63. Originally published online September 15, 2011 doi: 10.2522/ptj.20100167
Study.com 2016: What Is an Organ System? - Definition & Pictures
Chapter 14 / Lesson 13
Swenson 2002: Swenson KK, Nissen MJ, Ceronsky C, Swenson L, Lee MW & Tuttle TM: "Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancer" Ann Surg Oncol. 2002;9(8):745-53.
Szuba 1998: Szuba A & Rockson SG. “Lymphedema: classification, diagnosis and therapy” Vasc Med. 1998;3:145-156.
Torres-Lacomba 2010: Torres-Lacomba, M, Yuste-Sanchez MJ, Goni AZ, et al: "Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: Randomized, single blinded, clinical trial" BMJ 2010;340:b5396.
Tribute 2015: Solaris Product Information Sheet “TRIBUTENight Manufactured by Solaris —About TributeNight™ Therapeutic Garments” http://www.lymphedemadepot.com/infosheets/tribute.pdf [accessed 17 January 2015].
Weiss 2004-5: Weiss R: “Incidence of Lymphedema–A Literature Review” plenary presentation at the 6th International NLN Conference New Frontiers in Lymphedema Research & Therapy Reno, NV October 2004. Summary published in NLN LYMPHLink Jan-Mar 2005;17(1):12,27.
Weiss 2006: Weiss R. “Progress On The Medicare Coverage Front” NLN LymphLink Oct-Dec 2006;18(4):13.
Weiss 2007: Weiss R. "Three methods for the cost-efficacy analysis of lymphedema treatment" Poster Paper at American Society of Lymphology Annual Conference, Kansas City, MO, November 26-8, 2007.
Weiss 2016: Weiss R. “Cost of a lymphedema treatment mandate- 10 years of experience in the Commonwealth of Virginia” Health Economics Review (2016) 6:42 DOI 10.1186/s13561-016-0117-3.
Weissleder 2001: Weissleder H & Schuchhardt C Eds.: Lymphedema: Diagnosis and Therapy Third Edition, Viavital Verlag, Köln 2001: 187-91.
Yoshimoto 2004: Yoshimoto M, Tada K, Hori H, et al. “Improvement in the Prognosis of Japanese Breast Cancer Patients from 1946 to 2001—an Institutional Review” Jap J Clin Oncol. 2004;34(8):457-62.
Zimmermann 2012: Zimmerman A, Wozniewski M, Szklarska A, Lipowicz A and Szuba A. "Efficacy of manual lymphatic drainage in preventing secondary lymphedema after breast cancer surgery" Lymphology 2012; 45:103-12.
Zuther 2005: Zuther JE. Lymphedema Management. The comprehensive guide for practitioners Thieme, New York, Stuttgart, 2005:112-3.
Zuther 2014: Zuther JE. “The Science behind Compression Therapy in Lymphedema Management, Lymphedema” Inform Yourself and Take Control Blog, December 5, 2014.
APPENDICES
- 2005 change of HCPCS coding for compression stockings


Appendix B
- How Are Compression Bandages, Garments, Devices and Supplies Coverable under the Social Security Act? Robert Weiss, Revision 7, July 11, 2016, http://www.lymphactivist.org/prosthetic_devices-insert.pdf
- Proposed expansion of HCPCS Codes for Lymphedema Supplies
| CURRENT | DESCRIPTION |
|---|
|
Breast Prostheses |
|
L8000
|
Breast prosthesis, mastectomy bra
|
|
L8010
|
Breast prosthesis, mastectomy sleeve
|
|
L8015
|
External breast prosthesis garment, with mastectomy form, post mastectomy
|
|
|
|
|
|
Compression Garment, Lymphedema, Lower Limb
|
|
L8100/A6530
|
Compression Garment, Lymphedema, below knee, 18-30 mm Hg, each
|
|
L8110/A6531
|
Compression Garment, Lymphedema, below knee, 30-40 mm Hg, each
|
|
L8120/A6532
|
Compression Garment, Lymphedema, below knee, 40-50 mm Hg, each
|
|
L8130/A6533
|
Compression Garment, Lymphedema, thigh length, 18-30 mm Hg, each
|
|
L8140/A6534
|
Compression Garment, Lymphedema, thigh length, 30-40 mm Hg, each
|
|
L8150/A6535
|
Compression Garment, Lymphedema, thigh length, 40-50 mm Hg, each
|
|
L8160/A6536
|
Compression Garment, Lymphedema, full length/chap style, 18-30 mm Hg, each
|
|
L8170/A6537
|
Compression Garment, Lymphedema, full length/chap style, 30-40 mm Hg, each
|
|
L8180/A6538
|
Compression Garment, Lymphedema, full length/chap style, 40-50 mm Hg, each
|
|
L8190/A6539
|
Compression Garment, Lymphedema, waist length, 18-30 mm Hg, each
|
|
L8195/A6540
|
Compression Garment, Lymphedema, waist length, 30-40 mm Hg, each
|
|
L8200/A6541
|
Compression Garment, Lymphedema, waist length, 40-50 mm Hg, each
|
|
L8210/A6542
|
Compression Garment, Lymphedema, custom made
|
|
L8220/A6543
|
Compression Garment, Lymphedema, lymphedema
|
|
L8230/A6544
|
Compression Garment, Lymphedema, garter belt
|
|
L8239/A6549
|
Compression Garment, Lymphedema, not otherwise specified
|
|
|
Compression Garment, Lymphedema, foot
|
|
|
|
|
|
Compression Garment, Lymphedema, Upper Limb
|
|
S8420
|
Compression Garment, Lymphedema, sleeve and glove combination, custom made
|
|
S8421
|
Compression Garment, Lymphedema, sleeve and glove combination, ready made
|
|
S8422
|
Compression Garment, Lymphedema, sleeve, custom made, medium weight
|
|
S8423
|
Compression Garment, Lymphedema, sleeve, custom made, heavy weight
|
|
S8424/L8010
|
Compression Garment, Lymphedema, sleeve, ready made
|
|
S8425
|
Compression Garment, Lymphedema, glove, custom made, medium weight
|
|
S8426
|
Compression Garment, Lymphedema, glove, custom made, heavy weight
|
|
S8427
|
Compression Garment, Lymphedema, glove, ready made
|
|
S8428
|
Compression Garment, Lymphedema, gauntlet, ready made
|
|
S8429
|
Gradient pressure exterior wrap
|
|
S8430/A6421
|
Padding for compression bandage, roll
|
|
S8431/A64xx
|
Compression bandage, roll
|
|
|
|
|
|
Compression Garment, Lymphedema, Torso
|
|
|
Compression Garment, Lymphedema, upper torso
|
|
|
Compression Garment, Lymphedema, lower torso
|
|
|
|
|
|
Compression Garment, Lymphedema, Head and Neck
|
|
|
Compression Garment, Lymphedema, Head and Neck
|
|
|
|
|
|
Compression Binding Kit
|
|
|
Compression Binding Kit, Upper Limb, single
|
|
|
Compression Binding Kit, Upper Limb, double
|
|
|
Compression Binding Kit, Lower Limb, regular, single
|
|
|
Compression Binding Kit, Lower Limb, large single
|
|
|
Compression Binding Kit, Genital, male
|
|
|
Compression Binding Kit, Genital, female
|
|
|
Compression Binding Kit, Custom
|
|
|
Compression Binding Supplies
|
|
|
Compression Binding, Short-Stretch, 30-70% Extensibility, 5m roll, 4-6cm
|
|
|
Compression Binding, Short-Stretch, 30-70% Extensibility, 5m roll, 7-10cm
|
|
|
Compression Binding, Short-Stretch, 30-70% Extensibility, 5m roll, 11-14cm
|
|
|
Compression Binding, Short-Stretch, 30-70% Extensibility, 5m roll, 15-20cm
|
|
|
Compression Binding, Medium-Stretch, 75-100% Extensibility, 5m roll, 4-6cm
|
|
|
Compression Binding, Medium-Stretch, 75-100% Extensibility, 5m roll, 7-10cm
|
|
|
Compression Binding, Medium-Stretch, 75-100% Extensibility, 5m roll, 11-14cm
|
|
|
Compression Binding, Medium-Stretch, 75-100% Extensibility, 5m roll, 15-20cm
|
|
|
Compression Binding Gauze bandage, roll
|
|
A6457
|
Tubular Bandage, gauze, 20m roll, 2-6cm
|
|
|
Tubular Bandage, gauze, 20m roll, 6-12cm
|
|
|
Tubular Bandage, gauze, 20m roll, 15-20cm
|
|
|
Tubular Bandage, thick, 20m roll
|
|
|
Gauze Bandage, disposable, 4m roll, 2-6cm
|
|
|
Gauze Bandage, washable, 4m roll, 2-6cm
|
|
|
Padding, Cotton/Synthetic, Non-Woven, disposable, 3m roll, 6-10cm
|
|
|
Padding, Cotton/Synthetic, Non-Woven, disposable, 3m roll, 11-16cm
|
|
|
Padding, Cotton/Synthetic, Non-Woven, washable, 3m roll, 6-10cm
|
|
|
Padding, Cotton/Synthetic, Non-Woven, washable, 3m roll, 11-16cm
|
|
|
Padding, Soft Foam, ILD approx. 30, 2.5-3m roll, 6-10cm
|
|
|
Padding, Soft Foam, ILD approx. 30, 2.5-3m roll, 11-16cm
|
|
|
Foam, Synthetic Latex, Porous, ILD appr. 50, 10mm thick Sheet, 50x100cm
|
|
|
Foam, Synthetic Latex, Porous, ILD appr. 50, 5mm thick, 1m roll, 6-10cm
|
|
|
Foam, Synthetic Latex, Porous, ILD appr. 50, 5mm thick, 2m roll, 6-10cm
|
|
|
Foam, Synthetic Latex, Porous, ILD appr. 50, 10mm thick, 1m roll, 6-10cm
|
|
|
Foam, Synthetic Latex, Porous, ILD appr. 50, 10mm thick, 2m roll, 6-10cm
|
|
|
Foam, Synthetic Latex, Porous, ILD appr. 50, 5mm thick, shaped
|
|
|
Foam, Synthetic Latex, Porous, ILD appr. 50, 10-12mm thick, shaped
|
|
|
Foam, Synthetic Latex, Porous, mixed ILD, bag
|
|
|
Foam, Synthetic Latex, Porous, mixed ILD, mat
|
|
|
Foam, Synthetic Latex, Porous, ILD appr. 50, squares on mat
|
|
|
Bandage Winder, manual
|
|
|
Bandage Winder, powered
|
|
|
|
|
|
Lymphedema Directional Flow Garments and Pads
|
|
|
Directional Flow Garment with manually-adjustable compression, upper limb
|
|
|
Directional Flow Garment with elastic oversleeve, upper limb
|
|
|
Directional Flow Garment with manually-adjustable compression, lower limb
|
|
|
Directional Flow Garment with elastic oversleeve, lower limb
|
|
|
Directional Flow Garment, Lymphedema, upper limb
|
|
|
Directional Flow Garment, Lymphedema, hand
|
|
|
Directional Flow Garment, Lymphedema, lower limb
|
|
|
Directional Flow Garment, Lymphedema, foot
|
|
|
Directional Flow Pad, small (to 6 inches)
|
|
|
Directional Flow Pad, medium (6-12 inches)
|
|
|
Directional Flow Pad, large (over 12 inches)
|
|
|
Directional Flow Pad, ribbon
|
|
|
Compression Oversleeve, manually adjustable
|
|
|
Compression Oversleeve, elastic
|













